Unusual Cases in Genitourinary Pathology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Angiomyolipoma of the Cervix – Report of a Rare Entity
Internet Journal of Medical Update. 2017 July;12(2):13-15. doi: 10.4314/ijmu.v12i2.4 Internet Journal of Medical Update Journal home page: http://www.akspublication.com/ijmu Case Report Angiomyolipoma of the cervix – report of a rare entity N. Hariharanadha Sarmaᴪ1, Rama Srivastava2, Smriti Agnihotri3 1Consultant Pathologist, Department of Pathology, RDT Hospital, Bathalapalli, Andhra Pradesh, India 2Professor, Department of Pathology, SSR Medical College, Belle Rive, Mauritius 3Professor, Department of Pathology, American University of Antigua College of Medicine, Antigua & Barbuda, WI (Received 22 September 2017 and accepted 04 October 2017) ABSTRACT: Angiomyolipoma (AML) is a mesenchymal neoplasm seen usually in the kidney. Few cases of extra renal AML have been documented in various organs including the female genital tract, where the uterus is the most common site. To the best of our knowledge, only 4 cases of AML in the cervix have been reported in the literature. Association of AML with tuberous sclerosis is well known. Presently AML is included in the spectrum of disease entities called PEComa. We report a case of AML without tuberous sclerosis arising from the uterine cervix, which has to be differentiated from lipoleiomyoma. KEY WORDS: Angiomyolipoma; Uterine cervix; PEComas; Uterine tumor INTRODUCTIONV localization8, and mostly in females over 409. The occurrence of AML in the cervix without Angiomyolipoma (AML) occurs most frequently in concurrent incidence in the kidney is extremely the kidney, where it is closely related to tuberous rare, and only four cases have been reported in the sclerosis complex (TSC)1,2, occasionally in other scientific literature10. We are reporting a case of organs, most commonly the liver, but occurrence at uterine cervix AML without tuberous sclerosis other sites is extremely rare3. -

Soft Tissue Cytopathology: a Practical Approach Liron Pantanowitz, MD
4/1/2020 Soft Tissue Cytopathology: A Practical Approach Liron Pantanowitz, MD Department of Pathology University of Pittsburgh Medical Center [email protected] What does the clinician want to know? • Is the lesion of mesenchymal origin or not? • Is it begin or malignant? • If it is malignant: – Is it a small round cell tumor & if so what type? – Is this soft tissue neoplasm of low or high‐grade? Practical diagnostic categories used in soft tissue cytopathology 1 4/1/2020 Practical approach to interpret FNA of soft tissue lesions involves: 1. Predominant cell type present 2. Background pattern recognition Cell Type Stroma • Lipomatous • Myxoid • Spindle cells • Other • Giant cells • Round cells • Epithelioid • Pleomorphic Lipomatous Spindle cell Small round cell Fibrolipoma Leiomyosarcoma Ewing sarcoma Myxoid Epithelioid Pleomorphic Myxoid sarcoma Clear cell sarcoma Pleomorphic sarcoma 2 4/1/2020 CASE #1 • 45yr Man • Thigh mass (fatty) • CNB with TP (DQ stain) DQ Mag 20x ALT –Floret cells 3 4/1/2020 Adipocytic Lesions • Lipoma ‐ most common soft tissue neoplasm • Liposarcoma ‐ most common adult soft tissue sarcoma • Benign features: – Large, univacuolated adipocytes of uniform size – Small, bland nuclei without atypia • Malignant features: – Lipoblasts, pleomorphic giant cells or round cells – Vascular myxoid stroma • Pitfalls: Lipophages & pseudo‐lipoblasts • Fat easily destroyed (oil globules) & lost with preparation Lipoma & Variants . Angiolipoma (prominent vessels) . Myolipoma (smooth muscle) . Angiomyolipoma (vessels + smooth muscle) . Myelolipoma (hematopoietic elements) . Chondroid lipoma (chondromyxoid matrix) . Spindle cell lipoma (CD34+ spindle cells) . Pleomorphic lipoma . Intramuscular lipoma Lipoma 4 4/1/2020 Angiolipoma Myelolipoma Lipoblasts • Typically multivacuolated • Can be monovacuolated • Hyperchromatic nuclei • Irregular (scalloped) nuclei • Nucleoli not typically seen 5 4/1/2020 WD liposarcoma Layfield et al. -

Adenomatoid Tumor of the Skin: Differential Diagnosis of an Umbilical Erythematous Plaque
Title: Adenomatoid tumor of the skin: differential diagnosis of an umbilical erythematous plaque Keywords: Adenomatoid tumor, skin, umbilicus Short title: Adenomatoid tumor of the skin Authors: Ingrid Ferreira1, Olivier De Lathouwer2, Hugues Fierens3, Anne Theunis1, Josette André1, Nicolas de Saint Aubain3 1Dermatopathology laboratory, Department of Dermatology, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels, Belgium. 2Department of Plastic surgery, Centre Hospitalier Interrégional Edith Cavell, Waterloo, Belgium. 3Department of Dermatology, Saint-Jean Hospital, Brussels, Belgium. 4Department of Pathology, Jules Bordet Institute, Université Libre de Bruxelles, Brussels, Belgium. Acknowledgements: None Corresponding author: Ingrid Ferreira This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/cup.13872 This article is protected by copyright. All rights reserved. Abstract Adenomatoid tumors are benign tumors of mesothelial origin that are usually encountered in the genital tract. Although they have been observed in other organs, the skin appears to be a very rare location with only one case reported in the literature to our knowledge. We report a second case of an adenomatoid tumor, arising in the umbilicus of a 44-year-old woman. The patient presented with an 8 months old erythematous and firm plaque under the umbilicus. A skin biopsy showed numerous microcystic spaces dissecting a fibrous stroma and being lined by flattened to cuboidal cells with focal intraluminal papillary formation. This poorly known diagnosis constitutes a diagnostic pitfall for dermatopathologists and dermatologists, and could be misdiagnosed as other benign or malignant entities. -

The Health-Related Quality of Life of Sarcoma Patients and Survivors In
Cancers 2020, 12 S1 of S7 Supplementary Materials The Health-Related Quality of Life of Sarcoma Patients and Survivors in Germany—Cross-Sectional Results of A Nationwide Observational Study (PROSa) Martin Eichler, Leopold Hentschel, Stephan Richter, Peter Hohenberger, Bernd Kasper, Dimosthenis Andreou, Daniel Pink, Jens Jakob, Susanne Singer, Robert Grützmann, Stephen Fung, Eva Wardelmann, Karin Arndt, Vitali Heidt, Christine Hofbauer, Marius Fried, Verena I. Gaidzik, Karl Verpoort, Marit Ahrens, Jürgen Weitz, Klaus-Dieter Schaser, Martin Bornhäuser, Jochen Schmitt, Markus K. Schuler and the PROSa study group Includes Entities We included sarcomas according to the following WHO classification. - Fletcher CDM, World Health Organization, International Agency for Research on Cancer, editors. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press; 2013. 468 p. (World Health Organization classification of tumours). - Kurman RJ, International Agency for Research on Cancer, World Health Organization, editors. WHO classification of tumours of female reproductive organs. 4th ed. Lyon: International Agency for Research on Cancer; 2014. 307 p. (World Health Organization classification of tumours). - Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part B: Prostate and Bladder Tumours. Eur Urol. 2016 Jul;70(1):106–19. - World Health Organization, Swerdlow SH, International Agency for Research on Cancer, editors. WHO classification of tumours of haematopoietic and lymphoid tissues: [... reflects the views of a working group that convened for an Editorial and Consensus Conference at the International Agency for Research on Cancer (IARC), Lyon, October 25 - 27, 2007]. 4. ed. -

Non-Wilms Renal Cell Tumors in Children
PEDIATRIC UROLOGIC ONCOLOGY 0094-0143/00 $15.00 + .OO NON-WILMS’ RENAL TUMORS IN CHILDREN Bruce Broecker, MD Renal tumors other than Wilms’ tumor are tastases occur in 40% to 60% of patients with infrequent in childhood. Wilms’ tumors ac- clear cell sarcoma of the kidney, whereas they count for 6% to 7% of childhood cancer, are found in less than 2% of patients with whereas the remaining renal tumors account Wilms’ tumor.**,26 This distinct clinical behav- for less than l%.27The most common non- ior is one of the features that has led to its Wilms‘ tumors are clear cell sarcoma of the designation as a separate tumor. Other clini- kidney, rhabdoid tumor of the kidney (both cal features include a lack of association with formerly considered unfavorable Wilms’ tu- sporadic aniridia or hemihypertrophy. mor variants but now considered separate tu- Clear cell sarcoma of the kidney has not mors), renal cell carcinoma, mesoblastic been reported to occur bilaterally and is not nephroma, and multilocular cystic nephroma. associated with nephroblastomatosis. It has Collectively, these tumors account for less been reported in infancy and adulthood, but than 10% of the primary renal neoplasms in the peak incidence is between 3 and 5 years childhood. of age. It has an aggressive behavior that responds poorly to treatment with vincristine and actinomycin alone, leading to its original CLEAR CELL SARCOMA designation by Beckwith as an unfavorable histology pattern. The addition of doxorubi- Clear cell sarcoma of the kidney is cur- cin in aggressive chemotherapy regimens has rently considered a separate tumor distinct improved outcome. -
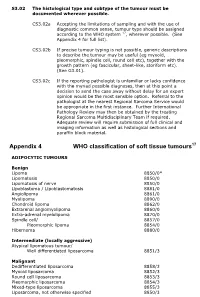
Appendix 4 WHO Classification of Soft Tissue Tumours17
S3.02 The histological type and subtype of the tumour must be documented wherever possible. CS3.02a Accepting the limitations of sampling and with the use of diagnostic common sense, tumour type should be assigned according to the WHO system 17, wherever possible. (See Appendix 4 for full list). CS3.02b If precise tumour typing is not possible, generic descriptions to describe the tumour may be useful (eg myxoid, pleomorphic, spindle cell, round cell etc), together with the growth pattern (eg fascicular, sheet-like, storiform etc). (See G3.01). CS3.02c If the reporting pathologist is unfamiliar or lacks confidence with the myriad possible diagnoses, then at this point a decision to send the case away without delay for an expert opinion would be the most sensible option. Referral to the pathologist at the nearest Regional Sarcoma Service would be appropriate in the first instance. Further International Pathology Review may then be obtained by the treating Regional Sarcoma Multidisciplinary Team if required. Adequate review will require submission of full clinical and imaging information as well as histological sections and paraffin block material. Appendix 4 WHO classification of soft tissue tumours17 ADIPOCYTIC TUMOURS Benign Lipoma 8850/0* Lipomatosis 8850/0 Lipomatosis of nerve 8850/0 Lipoblastoma / Lipoblastomatosis 8881/0 Angiolipoma 8861/0 Myolipoma 8890/0 Chondroid lipoma 8862/0 Extrarenal angiomyolipoma 8860/0 Extra-adrenal myelolipoma 8870/0 Spindle cell/ 8857/0 Pleomorphic lipoma 8854/0 Hibernoma 8880/0 Intermediate (locally -

The Identification of a Girl with Tuberous Sclerosis and an Unexpected Large Sized Renal Angiomyolipoma: a Case Report and Literature Review Study
Case Report Annals of Clinical Case Reports Published: 24 Aug, 2020 The Identification of a Girl with Tuberous Sclerosis and an Unexpected Large Sized Renal Angiomyolipoma: A Case Report and Literature Review Study Mitra Naseri1, Farah Ashrafzadeh2, Fatemeh Ghalibafan3* and Paria Dehghanian4 1Department of Pediatric Nephrology, Mashhad University of Medical Sciences, Iran 2Department of Pediatric Neurology, Mashhad University of Medical Sciences, Iran 3Department of Medicine, Mashhad University of Medical Sciences, Iran 4Department of Pathology, Mashhad University of Medical Sciences, Iran Abstract Background: The TSC1 mutated gene, hamartin, and the TSC2 gene, tuberin, cause tuberous sclerosis, the autosomal dominant tumor syndrome. The angiomyolipoma of the kidney is a benign tumor that often happens along with tuberous sclerosis. Large renal angiomyolipoma’s associated with tuberous sclerosis are in danger of fatal hemorrhage. This study reported a case with an unexpected large size of renal angiomyolipoma’s associated with tuberous sclerosis. Case Presentation: We report a girl aged 5 years and 9 months old with tuberous sclerosis who referred to nephrology clinic due to abdominal pain. Physical examination revealed a large mass in the right flank. Kidney ultrasound reported a large mass with approximate size of 90 mm × 60 mm in the right kidney, and abdominal CT scan also showed a non-hemogenic solid mass in the right kidney with size of 91 mm × 68 mm × 67 mm, was highly suspected for Wilms' tumor. Since OPEN ACCESS the large size -

Morphological and Immunohistochemical Characteristics of Surgically Removed Paediatric Renal Tumours in Latvia (1997–2010)
DOI: 10.2478/v10163-012-0008-6 ACTA CHIRURGICA LATVIENSIS • 2011 (11) ORIGINAL ARTICLE Morphological and Immunohistochemical Characteristics of Surgically Removed Paediatric Renal Tumours in Latvia (1997–2010) Ivanda Franckeviča*,**, Regīna Kleina*, Ivars Melderis** *Riga Stradins University, Riga, Latvia **Children’s Clinical University Hospital, Riga, Latvia Summary Introduction. Paediatric renal tumours represent 7% of all childhood malignancies. The variable appearances of the tumours and their rarity make them especially challenging group of lesions for the paediatric pathologist. In Latvia diagnostics and treatment of childhood malignancies is concentrated in Children’s Clinical University Hospital. Microscopic evaluation of them is realised in Pathology office of this hospital. Aim of the study is to analyze morphologic spectrum of children kidney tumours in Latvia and to characterise them from modern positions with wide range of immunohistochemical markers using morphological material of Pathology bureau of Children’s Clinical University Hospital. Materials and methods. We have analyzed surgically removed primary renal tumours in Children Clinical University Hospital from the year 1997 till 2010. Samples were fixed in 10% formalin fluid, imbedded in paraffin and haematoxylin-eosin stained slides were re-examined. Immunohistochemical re-investigation was made in 65.91% of cases. For differential diagnostic purposes were used antibodies for the detection of bcl-2, CD34, EMA, actin, desmin, vimentin, CKAE1/AE3, CK7, Ki67, LCA, WT1, CD99, NSE, chromogranin, synaptophyzin, S100, myoglobin, miogenin, MyoD1 (DakoCytomation) and INI1 protein (Santa Cruz Biotechnology). Results. During the revised period there were diagnosed 44 renal tumours. Accordingly of morphological examination data neoplasms were divided: 1) nephroblastoma – 75%, 2) clear cell sarcoma – 2.27%, 3) rhabdoid tumour – 4.55%, 4) angiomyolipoma – 4.55%, 5) embrional rhabdomyosarcoma – 2.27%, 6) mesoblastic nephroma – 4.55%, 7) multicystic nephroma – 4.55%, 8) angiosarcoma – 2.27%. -

Pediatric Abdominal Masses
Pediatric Abdominal Masses Andrew Phelps MD Assistant Professor of Pediatric Radiology UCSF Benioff Children's Hospital No Disclosures Take Home Message All you need to remember are the 5 common masses that shouldn’t go to pathology: 1. Infection 2. Adrenal hemorrhage 3. Renal angiomyolipoma 4. Ovarian torsion 5. Liver hemangioma Keys to (Differential) Diagnosis 1. Location? 2. Age? 3. Cystic? OUTLINE 1. Kidney 2. Adrenal 3. Pelvis 4. Liver OUTLINE 1. Kidney 2. Adrenal 3. Pelvis 4. Liver Renal Tumor Mimic – Any Age Infection (Pyelonephritis) Don’t send to pathology! Renal Tumor Mimic – Any Age Abscess Don’t send to pathology! Peds Renal Tumors Infant: 1) mesoblastic nephroma 2) nephroblastomatosis 3) rhabdoid tumor Child: 1) Wilm's tumor 2) lymphoma 3) angiomyolipoma 4) clear cell sarcoma 5) multilocular cystic nephroma Teen: 1) renal cell carcinoma 2) renal medullary carcinoma Peds Renal Tumors Infant: 1) mesoblastic nephroma 2) nephroblastomatosis 3) rhabdoid tumor Child: 1) Wilm's tumor 2) lymphoma 3) angiomyolipoma 4) clear cell sarcoma 5) multilocular cystic nephroma Teen: 1) renal cell carcinoma 2) renal medullary carcinoma Renal Tumors - Infant 1) mesoblastic nephroma 2) nephroblastomatosis 3) rhabdoid tumor Renal Tumors - Infant 1) mesoblastic nephroma 2) nephroblastomatosis 3) rhabdoid tumor - Most common - Can’t distinguish from congenital Wilms. Renal Tumors - Infant 1) mesoblastic nephroma 2) nephroblastomatosis 3) rhabdoid tumor Look for Multiple biggest or diffuse and masses. ugliest. Renal Tumors - Infant 1) mesoblastic -

Concurrent Hepatic Adenomatoid Tumor and Hepatic Hemangioma: a Case Report
pISSN 2287-2728 eISSN 2287-285X Case Report http://dx.doi.org/10.3350/cmh.2012.18.2.229 Clinical and Molecular Hepatology 2012;18:229-234 Concurrent hepatic adenomatoid tumor and hepatic hemangioma: a case report Ji-Beom Kim1, Eunsil Yu2, Ju-Hyun Shim1, Gi-Won Song3, Gwang Un Kim1, Young-Joo Jin1, and Ho-Seop Park2 1Department of Internal Medicine, 2Department of Pathology, and 3Division of Hepatobiliary Surgery and Liver Transplantation, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea A 45-year-old male with alleged asymptomatic hepatic hemangioma of 4 years duration had right upper-quadrant pain and was referred to a tertiary hospital. Computed tomography and magnetic resonance imaging scans revealed a hypervascular mass of about 7 cm containing intratumoral multilobulated cysts. A preoperative liver biopsy was performed, but this failed to provide a definitive diagnosis. The patient underwent a partial hepatectomy of segments IV and VIII. The histologic findings revealed multifocal proliferation of flattened or cuboidal epithelioid cells and a highly vascular edematous stroma. Immunohistochemistry findings demonstrated that the epithelioid tumor cells were positive for cytokeratin (AE1/AE3), vimentin, calretinin, and cytokeratin 5/6, and were focally positive for CD10, and negative for WT1 and CD34, all of which support their mesothelial origin. Immunohistochemistry for a mesothelial marker should be performed for determining the presence of an adenomatoid tumor when benign epithelioid -

About Soft Tissue Sarcoma Overview and Types
cancer.org | 1.800.227.2345 About Soft Tissue Sarcoma Overview and Types If you've been diagnosed with soft tissue sarcoma or are worried about it, you likely have a lot of questions. Learning some basics is a good place to start. ● What Is a Soft Tissue Sarcoma? Research and Statistics See the latest estimates for new cases of soft tissue sarcoma and deaths in the US and what research is currently being done. ● Key Statistics for Soft Tissue Sarcomas ● What's New in Soft Tissue Sarcoma Research? What Is a Soft Tissue Sarcoma? Cancer starts when cells start to grow out of control. Cells in nearly any part of the body can become cancer and can spread to other areas. To learn more about how cancers start and spread, see What Is Cancer?1 There are many types of soft tissue tumors, and not all of them are cancerous. Many benign tumors are found in soft tissues. The word benign means they're not cancer. These tumors can't spread to other parts of the body. Some soft tissue tumors behave 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 in ways between a cancer and a non-cancer. These are called intermediate soft tissue tumors. When the word sarcoma is part of the name of a disease, it means the tumor is malignant (cancer).A sarcoma is a type of cancer that starts in tissues like bone or muscle. Bone and soft tissue sarcomas are the main types of sarcoma. Soft tissue sarcomas can develop in soft tissues like fat, muscle, nerves, fibrous tissues, blood vessels, or deep skin tissues. -

Microrna Dysregulation Interplay with Childhood Abdominal Tumors
Cancer and Metastasis Reviews (2019) 38:783–811 https://doi.org/10.1007/s10555-019-09829-x MicroRNA dysregulation interplay with childhood abdominal tumors Karina Bezerra Salomão1 & Julia Alejandra Pezuk2 & Graziella Ribeiro de Souza1 & Pablo Chagas1 & Tiago Campos Pereira3 & Elvis Terci Valera1 & María Sol Brassesco3 Published online: 17 December 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Abdominal tumors (AT) in children account for approximately 17% of all pediatric solid tumor cases, and frequently exhibit embryonal histological features that differentiate them from adult cancers. Current molecular approaches have greatly improved the understanding of the distinctive pathology of each tumor type and enabled the characterization of novel tumor biomarkers. As seen in abdominal adult tumors, microRNAs (miRNAs) have been increasingly implicated in either the initiation or progression of childhood cancer. Moreover, besides predicting patient prognosis, they represent valuable diagnostic tools that may also assist the surveillance of tumor behavior and treatment response, as well as the identification of the primary metastatic sites. Thus, the present study was undertaken to compile up- to-date information regarding the role of dysregulated miRNAs in the most common histological variants of AT, including neuroblas- toma, nephroblastoma, hepatoblastoma, hepatocarcinoma, and adrenal tumors. Additionally, the clinical implications of dysregulated miRNAs as potential diagnostic tools or indicators of prognosis were evaluated. Keywords miRNA . Neuroblastoma . Nephroblastoma . Hepatoblastoma . Hepatocarcinoma . Adrenal tumors 1 MicroRNA biogenesis and function retaining the hairpin (pre-miRNA, ~ 70 nucleotides long), which is translocated by Exportin-5 to the cytoplasm, and then Fundamentally, all cellular programs are controlled by genes: processed by another endoribonuclease (Dicer) into the growth, senescence, division, metabolism, stemness, mobility, miRNA duplex.