Datasheet: VPA00701 Product Details
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Allele-Specific Expression of Ribosomal Protein Genes in Interspecific Hybrid Catfish
Allele-specific Expression of Ribosomal Protein Genes in Interspecific Hybrid Catfish by Ailu Chen A dissertation submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Auburn, Alabama August 1, 2015 Keywords: catfish, interspecific hybrids, allele-specific expression, ribosomal protein Copyright 2015 by Ailu Chen Approved by Zhanjiang Liu, Chair, Professor, School of Fisheries, Aquaculture and Aquatic Sciences Nannan Liu, Professor, Entomology and Plant Pathology Eric Peatman, Associate Professor, School of Fisheries, Aquaculture and Aquatic Sciences Aaron M. Rashotte, Associate Professor, Biological Sciences Abstract Interspecific hybridization results in a vast reservoir of allelic variations, which may potentially contribute to phenotypical enhancement in the hybrids. Whether the allelic variations are related to the downstream phenotypic differences of interspecific hybrid is still an open question. The recently developed genome-wide allele-specific approaches that harness high- throughput sequencing technology allow direct quantification of allelic variations and gene expression patterns. In this work, I investigated allele-specific expression (ASE) pattern using RNA-Seq datasets generated from interspecific catfish hybrids. The objective of the study is to determine the ASE genes and pathways in which they are involved. Specifically, my study investigated ASE-SNPs, ASE-genes, parent-of-origins of ASE allele and how ASE would possibly contribute to heterosis. My data showed that ASE was operating in the interspecific catfish system. Of the 66,251 and 177,841 SNPs identified from the datasets of the liver and gill, 5,420 (8.2%) and 13,390 (7.5%) SNPs were identified as significant ASE-SNPs, respectively. -

Anti-MRPS17 Monoclonal Antibody, Clone FQS23694 (DCABH-6110) This Product Is for Research Use Only and Is Not Intended for Diagnostic Use
Anti-MRPS17 monoclonal antibody, clone FQS23694 (DCABH-6110) This product is for research use only and is not intended for diagnostic use. PRODUCT INFORMATION Product Overview Rabbit monoclonal to MRPS17 Antigen Description Mammalian mitochondrial ribosomal proteins are encoded by nuclear genes and help in protein synthesis within the mitochondrion. Mitochondrial ribosomes (mitoribosomes) consist of a small 28S subunit and a large 39S subunit. They have an estimated 75% protein to rRNA composition compared to prokaryotic ribosomes, where this ratio is reversed. Another difference between mammalian mitoribosomes and prokaryotic ribosomes is that the latter contain a 5S rRNA. Among different species, the proteins comprising the mitoribosome differ greatly in sequence, and sometimes in biochemical properties, which prevents easy recognition by sequence homology. This gene encodes a 28S subunit protein that belongs to the ribosomal protein S17P family. The encoded protein is moderately conserved between human mitochondrial and prokaryotic ribosomal proteins. Pseudogenes corresponding to this gene are found on chromosomes 1p, 3p, 6q, 14p, 18q, and Xq. Immunogen Recombinant fragment within Human MRPS17. The exact sequence is proprietary.Database link: Q9Y2R5 Isotype IgG Source/Host Rabbit Species Reactivity Human Clone FQS23694 Purity Tissue culture supernatant Conjugate Unconjugated Applications IHC-P, WB, ICC/IF, IP Positive Control HeLa, HepG2 and U937 cell lysate; Human kidney and liver tissue; HeLa cells Format Liquid Size 100 μl Buffer pH: 7.2; Preservative: 0.01% Sodium azide; Constituents: 49% PBS, 0.05% BSA, 50% Glycerol 45-1 Ramsey Road, Shirley, NY 11967, USA Email: [email protected] Tel: 1-631-624-4882 Fax: 1-631-938-8221 1 © Creative Diagnostics All Rights Reserved Preservative 0.01% Sodium Azide Storage Store at +4°C short term (1-2 weeks). -

Supplementary File 2A Revised
Supplementary file 2A. Differentially expressed genes in aldosteronomas compared to all other samples, ranked according to statistical significance. Missing values were not allowed in aldosteronomas, but to a maximum of five in the other samples. Acc UGCluster Name Symbol log Fold Change P - Value Adj. P-Value B R99527 Hs.8162 Hypothetical protein MGC39372 MGC39372 2,17 6,3E-09 5,1E-05 10,2 AA398335 Hs.10414 Kelch domain containing 8A KLHDC8A 2,26 1,2E-08 5,1E-05 9,56 AA441933 Hs.519075 Leiomodin 1 (smooth muscle) LMOD1 2,33 1,3E-08 5,1E-05 9,54 AA630120 Hs.78781 Vascular endothelial growth factor B VEGFB 1,24 1,1E-07 2,9E-04 7,59 R07846 Data not found 3,71 1,2E-07 2,9E-04 7,49 W92795 Hs.434386 Hypothetical protein LOC201229 LOC201229 1,55 2,0E-07 4,0E-04 7,03 AA454564 Hs.323396 Family with sequence similarity 54, member B FAM54B 1,25 3,0E-07 5,2E-04 6,65 AA775249 Hs.513633 G protein-coupled receptor 56 GPR56 -1,63 4,3E-07 6,4E-04 6,33 AA012822 Hs.713814 Oxysterol bining protein OSBP 1,35 5,3E-07 7,1E-04 6,14 R45592 Hs.655271 Regulating synaptic membrane exocytosis 2 RIMS2 2,51 5,9E-07 7,1E-04 6,04 AA282936 Hs.240 M-phase phosphoprotein 1 MPHOSPH -1,40 8,1E-07 8,9E-04 5,74 N34945 Hs.234898 Acetyl-Coenzyme A carboxylase beta ACACB 0,87 9,7E-07 9,8E-04 5,58 R07322 Hs.464137 Acyl-Coenzyme A oxidase 1, palmitoyl ACOX1 0,82 1,3E-06 1,2E-03 5,35 R77144 Hs.488835 Transmembrane protein 120A TMEM120A 1,55 1,7E-06 1,4E-03 5,07 H68542 Hs.420009 Transcribed locus 1,07 1,7E-06 1,4E-03 5,06 AA410184 Hs.696454 PBX/knotted 1 homeobox 2 PKNOX2 1,78 2,0E-06 -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Micrornas Mediated Regulation of the Ribosomal Proteins and Its Consequences on the Global Translation of Proteins
cells Review microRNAs Mediated Regulation of the Ribosomal Proteins and Its Consequences on the Global Translation of Proteins Abu Musa Md Talimur Reza 1,2 and Yu-Guo Yuan 1,3,* 1 Jiangsu Co-Innovation Center of Prevention and Control of Important Animal Infectious Diseases and Zoonoses, College of Veterinary Medicine, Yangzhou University, Yangzhou 225009, China; [email protected] 2 Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Pawi´nskiego5a, 02-106 Warsaw, Poland 3 Jiangsu Key Laboratory of Zoonosis/Joint International Research Laboratory of Agriculture and Agri-Product Safety, The Ministry of Education of China, Yangzhou University, Yangzhou 225009, China * Correspondence: [email protected]; Tel.: +86-514-8797-9228 Abstract: Ribosomal proteins (RPs) are mostly derived from the energy-consuming enzyme families such as ATP-dependent RNA helicases, AAA-ATPases, GTPases and kinases, and are important structural components of the ribosome, which is a supramolecular ribonucleoprotein complex, composed of Ribosomal RNA (rRNA) and RPs, coordinates the translation and synthesis of proteins with the help of transfer RNA (tRNA) and other factors. Not all RPs are indispensable; in other words, the ribosome could be functional and could continue the translation of proteins instead of lacking in some of the RPs. However, the lack of many RPs could result in severe defects in the biogenesis of ribosomes, which could directly influence the overall translation processes and global expression of the proteins leading to the emergence of different diseases including cancer. While microRNAs (miRNAs) are small non-coding RNAs and one of the potent regulators of the post-transcriptional 0 gene expression, miRNAs regulate gene expression by targeting the 3 untranslated region and/or coding region of the messenger RNAs (mRNAs), and by interacting with the 50 untranslated region, Citation: Reza, A.M.M.T.; Yuan, Y.-G. -

Mitochondrial Translation and Its Impact on Protein Homeostasis And
Mitochondrial translation and its impact on protein homeostasis and aging Tamara Suhm Academic dissertation for the Degree of Doctor of Philosophy in Biochemistry at Stockholm University to be publicly defended on Friday 15 February 2019 at 09.00 in Magnélisalen, Kemiska övningslaboratoriet, Svante Arrhenius väg 16 B. Abstract Besides their famous role as powerhouse of the cell, mitochondria are also involved in many signaling processes and metabolism. Therefore, it is unsurprising that mitochondria are no isolated organelles but are in constant crosstalk with other parts of the cell. Due to the endosymbiotic origin of mitochondria, they still contain their own genome and gene expression machinery. The mitochondrial genome of yeast encodes eight proteins whereof seven are core subunits of the respiratory chain and ATP synthase. These subunits need to be assembled with subunits imported from the cytosol to ensure energy supply of the cell. Hence, coordination, timing and accuracy of mitochondrial gene expression is crucial for cellular energy production and homeostasis. Despite the central role of mitochondrial translation surprisingly little is known about the molecular mechanisms. In this work, I used baker’s yeast Saccharomyces cerevisiae to study different aspects of mitochondrial translation. Exploiting the unique possibility to make directed modifications in the mitochondrial genome of yeast, I established a mitochondrial encoded GFP reporter. This reporter allows monitoring of mitochondrial translation with different detection methods and enables more detailed studies focusing on timing and regulation of mitochondrial translation. Furthermore, employing insights gained from bacterial translation, we showed that mitochondrial translation efficiency directly impacts on protein homeostasis of the cytoplasm and lifespan by affecting stress handling. -

The Role of Human Ribosomal Proteins in the Maturation of Rrna and Ribosome Production
JOBNAME: RNA 14#9 2008 PAGE: 1 OUTPUT: Friday August 8 17:34:50 2008 csh/RNA/164293/rna11320 Downloaded from rnajournal.cshlp.org on September 27, 2021 - Published by Cold Spring Harbor Laboratory Press The role of human ribosomal proteins in the maturation of rRNA and ribosome production SARA ROBLEDO,1,3 RACHEL A. IDOL,1,3 DAN L. CRIMMINS,2 JACK H. LADENSON,2 PHILIP J. MASON,1,4 and MONICA BESSLER1,4 1Department of Internal Medicine, Division of Hematology, Washington University School of Medicine, St. Louis, Missouri 63110, USA 2Department of Pathology and Immunology, Division of Laboratory and Genomic Medicine, Washington University School of Medicine, St. Louis, Missouri 63110, USA ABSTRACT Production of ribosomes is a fundamental process that occurs in all dividing cells. It is a complex process consisting of the coordinated synthesis and assembly of four ribosomal RNAs (rRNA) with about 80 ribosomal proteins (r-proteins) involving more than 150 nonribosomal proteins and other factors. Diamond Blackfan anemia (DBA) is an inherited red cell aplasia caused by mutations in one of several r-proteins. How defects in r-proteins, essential for proliferation in all cells, lead to a human disease with a specific defect in red cell development is unknown. Here, we investigated the role of r-proteins in ribosome biogenesis in order to find out whether those mutated in DBA have any similarities. We depleted HeLa cells using siRNA for several individual r-proteins of the small (RPS6, RPS7, RPS15, RPS16, RPS17, RPS19, RPS24, RPS25, RPS28) or large subunit (RPL5, RPL7, RPL11, RPL14, RPL26, RPL35a) and studied the effect on rRNA processing and ribosome production. -
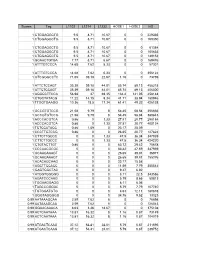
Scores Tag L1102 L1214 L1232 HOSE1 HOSE2 HS 1
Scores Tag L1102 L1214 L1232 HOSE1 HOSE2 HS 1 CTGGAGGCTG 9.5 8.71 10.67 0 0 229335 1 CTGGAGGCTG 9.5 8.71 10.67 0 0 169350 1 CTGGAGGCTG 9.5 8.71 10.67 0 0 61384 1 CTGGAGGCTG 9.5 8.71 10.67 0 0 105633 1 CTGGAGGCTG 9.5 8.71 10.67 0 0 149152 1 GCAACTGTGA 7.77 8.71 6.67 0 0 169476 1 ATTTGTCCCA 14.68 7.62 5.33 0 0 57301 1 ATTTGTCCCA 14.68 7.62 5.33 0 0 356122 1 GTCGGGCCTC 71.65 39.18 22.67 1.16 0 73769 1 ATTCTCCAGT 35.39 39.18 44.01 85.74 89.13 458218 1 ATTCTCCAGT 35.39 39.18 44.01 85.74 89.13 406300 1 AGGGCTTCCA 56.98 37 69.35 134.4 141.05 458148 1 CTGCTATACG 11.22 14.15 9.34 41.71 38.94 180946 1 TTGGTGAAGG 10.36 18.5 17.34 61.41 49.32 426138 1 GCCGTGTCCG 21.58 9.79 8 54.45 58.84 356666 1 GCCGTGTCCG 21.58 9.79 8 54.45 58.84 380843 1 ACCCACGTCA 0.86 0 1.33 27.81 20.77 298184 1 ACCCACGTCA 0.86 0 1.33 27.81 20.77 400124 1 TCTCCATACC 0.86 1.09 0 23.17 25.09 1 CCCTTGTCCG 0.86 0 0 26.65 20.77 127824 1 CTTCTTGCCC 0 0 1.33 47.5 36.34 347939 1 CTTCTTGCCC 0 0 1.33 47.5 36.34 424220 1 CTGTACTTGT 0.86 0 0 63.72 29.42 75678 1 CCCAACGCGC 0 0 0 83.42 47.59 347939 1 GCAAGAAAGT 0 0 0 26.65 39.81 36977 1 GCAAGAAAGT 0 0 0 26.65 39.81 155376 1 ACACAGCAAG 0 0 0 23.17 15.58 1 AGCTTCCACC 0 0 0 11.59 7.79 355542 1 GAGTGGCTAC 0 0 0 9.27 6.92 1 ATGGTGGGGG 0 0 0 8.11 22.5 343586 1 AGATCCCAAG 0 0 0 5.79 8.65 50813 1 TGGAAGGAGG 0 0 0 8.11 6.06 1 TAGCCGGGAC 0 0 0 5.79 7.79 107740 1 TGTGGATGTG 0 0 0 4.63 12.11 180878 1 GGGTAGGGGG 0 0 0 34.76 9.52 13323 0.99 AATAAAGCAA 2.59 7.62 8 0 0 76698 0.99 AATAAAGCAA 2.59 7.62 8 0 0 126043 0.99 GGAACAAACA 8.63 3.26 18.67 0 0 375108 -

RPSA Gene Ribosomal Protein SA
RPSA gene ribosomal protein SA Normal Function The RPSA gene provides instructions for making a protein called ribosomal protein SA, which is one of approximately 80 different ribosomal proteins. These proteins come together to form structures called ribosomes. Ribosomes process the cell's genetic instructions to create proteins. Each ribosome is made up of two parts (subunits) called the large subunit and the small subunit. Ribosomal protein SA is part of the small subunit. The specific roles of each of the ribosomal proteins within the ribosome are not entirely understood. Some ribosomal proteins are involved in the assembly or stability of ribosomes. Others help carry out the ribosome's main function of building new proteins. Research suggests that ribosomal protein SA helps the ribosome control the production of certain proteins, many of which are likely important for development before birth. Health Conditions Related to Genetic Changes Isolated congenital asplenia At least 20 RPSA gene mutations have been identified in individuals with isolated congenital asplenia. People with this condition do not have a spleen but have no other developmental abnormalities. The spleen plays an important role in the immune system. Without this organ, affected individuals are highly susceptible to bacterial infections, which can be life-threatening. RPSA gene mutations are thought to reduce the amount of functional ribosomal protein SA. A shortage of the normal protein likely impairs the assembly of ribosomes, but the specific effects of the mutations -

Ribosomal Proteins Expression and Phylogeny in Alpaca (Lama Pacos) Skin*
Advances in Bioscience and Biotechnology, 2012, 3, 231-237 ABB http://dx.doi.org/10.4236/abb.2012.33032 Published Online June 2012 (http://www.SciRP.org/journal/abb/) Ribosomal proteins expression and phylogeny in alpaca * (Lama pacos) skin Junming Bai, Ruiwen Fan, Shanshan Yang, Yuankai Ji, Jianshan Xie, Chang-Sheng Dong# College of Animal Science and Veterinary Medicine, Shanxi Agricultural University, Taigu, China Email: #[email protected] Received 3 February 2012; revised 8 March 2012; accepted 24 April 2012 ABSTRACT folding/chaperone role in facilitating the processing and folding of rRNA during biogenesis and stabilizing the Ribosomal proteins (RP) has been reported as a cen- mature particle during protein synthesis [3]. It has been tral player in the translation system, and are required well established that global regulation of protein synthe- for the growth and maintenance of all cell kinds. RP sis in eukaryotes is mainly achieved by posttranslational genes form a family of homologous proteins that ex- modification (PTM) of translation factors in response to press in the stable pattern and were used for phy- environmental cues [4]. logenetic analysis. Here we constructed a cDNA li- The family of South American camelids with four brary of alpaca skin and 13,800 clones were sequenced. members is recognized as two wild species, the gua- In the cDNA library, RP genes from skin cDNA li- naco (Lama guanicoe) and the vicuna (Vicugna vicugna) brary of alpaca were identified. Then 8 RP genes were and two of domestic species, the alpaca (Lama pacos) selected at random and built the phylogenetic trees and the llama [5]. -

Inflammation Leads to Distinct Populations of Extracellular Vesicles from Microglia Yiyi Yang1* , Antonio Boza-Serrano1, Christopher J
Yang et al. Journal of Neuroinflammation (2018) 15:168 https://doi.org/10.1186/s12974-018-1204-7 RESEARCH Open Access Inflammation leads to distinct populations of extracellular vesicles from microglia Yiyi Yang1* , Antonio Boza-Serrano1, Christopher J. R. Dunning2, Bettina Hjelm Clausen3,4, Kate Lykke Lambertsen3,4,5 and Tomas Deierborg1* Abstract Background: Activated microglia play an essential role in inflammatory responses elicited in the central nervous system (CNS). Microglia-derived extracellular vesicles (EVs) are suggested to be involved in propagation of inflammatory signals and in the modulation of cell-to-cell communication. However, there is a lack of knowledge on the regulation of EVs and how this in turn facilitates the communication between cells in the brain. Here, we characterized microglial EVs under inflammatory conditions and investigated the effects of inflammation on the EV size, quantity, and protein content. Methods: We have utilized western blot, nanoparticle tracking analysis (NTA), and mass spectrometry to characterize EVs and examine the alterations of secreted EVs from a microglial cell line (BV2) following lipopolysaccharide (LPS) and tumor necrosis factor (TNF) inhibitor (etanercept) treatments, or either alone. The inflammatory responses were measured with multiplex cytokine ELISA and western blot. We also subjected TNF knockout mice to experimental stroke (permanent middle cerebral artery occlusion) and validated the effect of TNF inhibition on EV release. Results: Our analysis of EVs originating from activated BV2 microglia revealed a significant increase in the intravesicular levels of TNF and interleukin (IL)-6. We also observed that the number of EVs released was reduced both in vitro and in vivo when inflammation was inhibited via the TNF pathway. -

Elabscience.Com ® E-Mail:[email protected] Elabscience Elabscience Biotechnology Inc
Tel:240-252-7368(USA) Fax:240-252-7376(USA) www.elabscience.com ® E-mail:[email protected] Elabscience Elabscience Biotechnology Inc. MRPL20 Polyclonal Antibody Catalog No. E-AB-18777 Reactivity H,M Storage Store at -20℃. Avoid freeze / thaw cycles. Host Rabbit Applications WB,IHC,ELISA Isotype IgG Note: Centrifuge before opening to ensure complete recovery of vial contents. Images Immunogen Information Immunogen Fusion protein of human MRPL20 Gene Accession BC009515 Swissprot Q9BYC9 Synonyms 39S ribosomal protein L20,mitochondrial,L20mt,MG C4779,MGC74465,Mitochondrial ribosomal protein L20,MRPL 20 Western blot analysis of RAW264.7 Product Information cell lysate using MRPL20 Polyclonal Calculated MW 17 kDa Antibody at dilution of 1:900 Observed MW Refer to figures Buffer PBS with 0.05% NaN3 and 40% Glycerol,pH7.4 Purify Antigen affinity purification Dilution WB 1:500-1:2000, IHC 1:50-1:200, ELISA 1:5000-1:10000 Background MRPL20 is one of more than 70 protein components of mitochondrial Immunohistochemistry of paraffin- ribosomes that are encoded by the nuclear genome. MRPL20 is a subunit embedded Human liver cancer tissue of the 39S mitochondrial ribosome. Mitochondrial ribosomes using MRPL20 Polyclonal Antibody at (mitoribosomes) consist of a small 28S subunit and a large 39S subunit. dilution of 1:60(×200) They have an estimated 75% protein to rRNA composition compared to prokaryotic ribosomes, where this ratio is reversed. Another difference between mammalian mitoribosomes and prokaryotic ribosomes is that the latter contain a 5S rRNA. Among different species, the proteins comprising the mitoribosome differ greatly in sequence, and sometimes in biochemical properties, which prevents easy recognition by sequence homology.