Theory of Staining Prepared By: Ms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Staining Wood and Natural Materials Using PRO Washfast Water Based
Staining Wood and Natural Materials using PRO WashFast Water-Based Dyes Please read directions carefully before starting. Many materials, whose surface is only slightly wettable, may be colored with water-based (water soluble) dyes resulting in colored surfaces that do not hide the grain of the wood or the character of the substrate. This process is also suitable for the coloration of feathers, retouching wool tapestries and wool or nylon carpeting. Basically anything that does not have the high wash fastness requirements of wearable clothing fabrics. Wet fastness is good. Always do test samples before working on a large project. For additional information visit our web site at www.prochemicalanddye.com. Wear rubber gloves, apron, or old clothes. Utensils used for dyeing should never be used for food preparation. Procedure: 1. Dissolve the PRO WashFast Acid dye by placing 1 tsp (2.5 gm) of the dye in a one cup (250 ml) Pyrex container. Add 1 or 2 tsp (5 or 10 ml) of boiling water to make a paste. Gradually add more boiling water with continuous stirring to the 2 cup (125 ml) mark. Set aside to cool. 2. When cooled to room temperature, fill to one cup (250 ml) mark with denatured grain alcohol or Isopropyl rubbing alcohol (90%). Store in re-sealable glass container. 3. Apply to surface of substrate with foam brush or immerse object into dye solution for several minutes. Air dry and repeat procedure as often as needed for deeper color allowing previous coat to dry thoroughly. 4. After thorough drying, wood and similar materials may be coated to seal in the color and give lasting protection to the wood itself. -

Fulltext Download
J. Indonesian Trop. Anim. Agric. 39(3):188-193, September 2014 ISSN 2087-8273 THE CHROME-TANNED GOAT LEATHER FOR HIGH QUALITY OF BATIK W. Pancapalaga1,2, V. P. Bintoro1, Y. B. Pramono1 and S. Triatmojo3 1Doctorate Program of Animal Science, Faculty of Animal and Agricultural Sciences, Diponegoro University, Tembalang Campus, Semarang 50275 - Indonesia 2Permanent Address: Faculty of Agriculture and Animal Sciences, Malang Muhammadiyah University, Jl. Raya Tlogomas No. 246, Malang 65148 - Indonesia 3Faculty of Animal Science, Gajah Mada University, Jl. Fauna, Bulaksumur, Yogyakarta 55281 - Indonesia Corresponding E-mail: [email protected] Received July 03, 2014; Accepted August 24, 2014 ABSTRAK Penelitian bertujuan untuk mengevaluasi kualitas batik kulit yang disamak dengan krom. Penelitian dilakukan secara bertahap dengan tahap pertama bertujuan untuk mengevaluasi natrium silika sebagai bahan pelepas lilin batik pada kulit samak krom. Penelitian menggunakan rancangan acak lengkap (RAL) sebagai perlakuan adalah kosentrasi natrium silika yaitu P1 = 0 , P2 = 2 g/l, P3 = 4 g/L dan P4 = 6 g/L diulang 9 kali. Penelitian tahap kedua bertujuan untuk mengevaluasi jenis bahan warna yang digunakan dalam pewarnaan metode batik pada kulit kambing samak krom. Penelitian menggunakan rancangan acak lengkap (RAL) sebagai perlakuan adalah jenis bahan pewarna yaitu P'1 = asam , P'2 = indigosol, P'3 = napthol dan P'4 = remazol diulang 9 kali. Berdasarkan hasil penelitian penggunaan natrium silika kosentrasi 2 g/L menghasilkan prosentasi lilin yang terlepas sebesar 91,4 % serta tidak menurunkan kualitas kulit samak krom. Jenis bahan warna asam dan napthol memberikan kuat rekat dan kecerahan warna terbaik serta ketahanan cuci, air, keringat, tekuk dan gosok yang terbaik yaitu 4/5 sampai 5 pada skala abu abu. -

CIBA Acid F.Pdf
./ Fiber Types · Safety InUse• Ciba Washfast Acid Dyes may be used on the Although no chemical is entirely freefrom hazard, · following fiber types: these products will pres�nt a low to no health risk, • Wool (includirg Cashmere, Alpaca, Angora, provided that good standards of studio· hygiene are and other protein fi�rs) observed in their use and storage. All persons. • Silk handling them should take precautions to avoid Techniques• Nylon· accidental ingestion, inhalation, skin and eye contact and should be aware of any limitations of use of specific products. While dyes and the • high temperature immersion chemicals associated with their use are not highly • handpainting silkscreening ,. toxic, they are industrtal chemicals and should be • block prtnting handled with care. Chemical productsshould not • airbrushing be allowed to get into the eyes, but 1f they should • warp painting by accident, wash eyes with clean water and then_ • resist (paste resist, gutta, bound) obtain medical treatment. Prolonged or repeated • batch dyeing (tie dyeing, rainbow dyeing) contact with skin should be avoided. Wear rubber gloves and use implements to stir solutions and ColorSeereverse Availablefor further deta ils.· dyebaths. Inhalation of'dusts .should be avoided by careful handling of powders. If the dyes are handled where particles may become airborne, a Yellow, Gold Yellow, Scarlet, Fuchsia, Turquoise, suitable dust respirator should be worn. Navy, Brown, Black, Green, Blue, and Violet. Obviously, chemicals slJ.ould ,not be taken -WhatNote: These Youdyes- Willare Need completely intennixable. internally, and the use of food, drink and smoking materials should be prohibited where chemicals are employed. The utensils used fordyeing should Stainless steel, enamel, plastic or glass measuring. -

Dyeing of Polyamide Fibres
Dyeing of Polyamide Fibres Polyamides • Nylon a Polyamide, it is a condensation polymers. The formation of a polyamide is same as the synthesis of a simple amide. One prominent difference is that both the amine and the acid monomer units each have two functional groups ‐ one on each side of the molecule. In this polymer, the repeating units are identical. • Nylon is made from 1,6‐diaminohexane and adipic acid by elimination of water molecules (‐H from the amine and ‐OH from acid as shown in red on the graphic). • A simple representation is ‐[A‐B‐A‐B‐A‐B]‐. Nylon 66 • Nylon 66, was discovered in 1931 by Wallace Cruthers at DuPont. It was the first fully synthetic fiber produced. It was introduced to women in nylon stockings in 1939 with huge success. During World War II, nylon production was used to make parachutes and other items needed by the military. • Nylon is very similar in structure to the protein polyamides in silk and wool as shown earlier, but is far stronger, more durable, more chemically inert, and cheaper to produce as compared to the natural fibers. Polyamide Fibres • It’s a Nylon fibre we generally know. • It consists of multiples of six carbon chains, in which half the end of carbon being converted to carbonyl and other half to imino nitrogen. • It is thermoplastic , is sensitive to heat and tension applied in various texturizing processes. • Total temperature‐tension history of yarn determines the degree of orientation in textured yarns. Dyeing of Polyamide Fibres • Acid, metal complexes, disperse reactive and disperse dyes are the important classes of dyes used in dyeing of nylon. -

Troubleshooting H&E Stain
Melinda M. Chow, MS, HT(ASCP)CM Memorial Sloan Kettering Cancer Center Basking Ridge, New Jersey Hematoxylin & Eosin staining is the most frequent routine stain in the Mohs Micrographic Surgery tissue preparation. It has stood the test of time as the standard stain for histologic examination of human tissues since it was independently introduced in 1865 and 1875, by Böhmer and Fischer respectively. Common problems, pitfalls and troubleshooting tips. H & E is the primary diagnostic technique for evaluation of morphology in the histopathology labs. One of the best nuclear stains. H & E provides easier identification of histological features than T-blue. It is easy and simple to use. Stains are inexpensive, yet reliable and informative. It is stable and durable stain, lasting years without fading Hematoxylin is a natural dye extracted from the heartwood of logwood trees which is indigenous in Central America, Caribbean and other tropical countries. It is misleading to call hematoxylin stain as it alone does not stain. It has to convert to hematein. Hematein is what we call hematoxylin. It is a basic dye and carries a (+) charge. Affinity for basic dye is called basophilic. Hematein Chromatin (+) charge (-) charge Mordant (Al+3,Fe+3,Chr+3) This complex is held by covalent bonds Hematein-mordant-chromatin complex Courtesy of Biotek Progressive vs regressive Progressive stains are: Gill’s (I-III), Mayer’s Regressive stains are Harris's, Delafield's, Ehrlich's Progressive method : tissue is stained and stopped Regressive method: tissue is overstained Eosin is a synthetic stain It is the counterstain and acts as an acid dye. -

Welcome to Arrowmont 3
WELCOME TO ARROWMONT 3 “Te most regretful people on earth are those who felt the call to creative work… and gave to it neither the power nor time.” Mary Oliver IMPORTANT DATES AT A GLANCE Whether for you it is creative work or creative play, if you will make the time, Arrowmont will provide the place, the opportunity, and the encouragement. ARTISTS-IN-RESIDENCE Arrowmont’s commitment to education and appreciation of crafts is built upon its APPLICATION DEADLINE heritage as a settlement school founded by Pi Beta Phi in 1912. Our 13 acre campus February 1, 2017 has six buildings on the National Register of Historic Places, well-equipped studios, and places for contemplation and conversation. We appreciate being described as a EARLY REGISTRATION DEADLINE “hidden jewel” and a “neighbor” of Great Smoky Mountains National Park. REGISTRATION FEE OF $50 IS WAIVED FOR EARLY REGISTRATION Here, you will spend time immersed in the studio, you will also eat well, have the February 1, 2017 opportunity to enjoy our library, and be inspired by our galleries. EDUCATIONAL Each week a new creative community forms. Students do more than participate in this ASSISTANTS PROGRAM community, they often develop lifelong relationships. Teir shared experiences of refecting, APPLICATION DEADLINE problem solving and making creates the community. Workshops are taught by some March 1, 2017 of the fnest artists from around the world — it is their commitment to sharing their SCHOLARSHIP APPLICATION knowledge and their experiences that makes them great teachers. Tey recognize that DEADLINE there is always more to learn, and that the environment of small groups of students, March 1, 2017 engaged in experimenting and discovering together, is both inspiring and energizing. -

Hematoxylin Formulae
Hematoxylin Formulae Bryan D Llewellyn Produced for distribution through StainsFile The Internet Resource For Histotechnologists http://stainsfile.info This document includes formulae for the alum and iron hematoxylin solutions included on the StainsFile Internet site. Some of these are no longer in use, and others are variations of other, more common, formulae. I have included a dis- cussion of the relationship between the dye and the mordant, and how the rela- tionship between them affects the staining character of a formula. The formulae have been collected from several sources, including older reference texts and the Internet. If any reader knows of a hematoxylin formula not listed in this document, I would most appreciate receiving details so that it may be included in the future. I may be contacted by e-mail through the StainsFile Internet site. Bryan D. Llewellyn October, 2013 October 2013 1.0 Initial document This document may be freely reproduced and distributed for educational pur- poses provided that the text is unchanged and no charge is made. Commercial distribution is not permitted. October, 2013. © 2013, Bryan D. Llewellyn. Table of Contents Hematoxylin and Hematein ............... 4 Oxidation ........................................... 6 Mordant ............................................. 9 Reactions With Tissue ...................... 12 Staining Mucin ................................... 13 Dye:Mordant Ratio ............................ 14 Acids ................................................. 19 Differentiation ................................... -

The Textile Museum Thesaurus
The Textile Museum Thesaurus Edited by Cecilia Gunzburger TM logo The Textile Museum Washington, DC This publication and the work represented herein were made possible by the Cotsen Family Foundation. Indexed by Lydia Fraser Designed by Chaves Design Printed by McArdle Printing Company, Inc. Cover image: Copyright © 2005 The Textile Museum All rights reserved. No part of this document may be reproduced, stored in a retrieval system, or transmitted in any form or by any means -- electronic, mechanical, photocopying, recording or otherwise -- without the express written permission of The Textile Museum. ISBN 0-87405-028-6 The Textile Museum 2320 S Street NW Washington DC 20008 www.textilemuseum.org Table of Contents Acknowledgements....................................................................................... v Introduction ..................................................................................................vii How to Use this Document.........................................................................xiii Hierarchy Overview ....................................................................................... 1 Object Hierarchy............................................................................................ 3 Material Hierarchy ....................................................................................... 47 Structure Hierarchy ..................................................................................... 55 Technique Hierarchy .................................................................................. -

ABSTRACT YI, DING. a Comparison of Mordant and Natural Dyes In
ABSTRACT YI, DING. A Comparison of Mordant and Natural Dyes in Dyeing Cotton Fabrics. (Under the direction of Dr. Harold S. Freeman and Dr. Peter J. Hauser). Mordant dyes constitute a class of synthetic colorants that are applied to textile fibers mainly through the aid of transition metal ions such as Cr3+. The resultant dye-metal complexes are key to the fastness properties produced on wool. In the same way, most natural dyes require the use of a mordant to have coloring power on textiles such as cotton. Unlike mordant dyes on wool, the fastness properties of natural dyes on cotton are generally quite low. Consequently, they have largely been replaced by synthetic dyes that are more cost effective, brighter, and more durable under end-use applications. Interest in green technologies has led to renewed consideration of natural dyes for textile coloration because they are biodegradable and do not involve manufacturing processes requiring genotoxic aromatic amines. Interestingly, such a shift would bring dyeing technology full circle to a family of colorants lacking the vibrancy and technical properties of most synthetic dyes. However, similarities between the dyeing method for mordant and natural dyes brought to mind the potential for reducing the level of synthetic dyes used commercially by combining suitable dyes from these two classes for dyeing textiles. This thesis research was devoted to stage 1 of this idea, namely the evaluation of mordant dyes as colorants for cotton fabric. With this in mind, dyes such as Mordant Blue 13, Mordant Brown 40, Mordant Orange 6 and Mordant Yellow 8 were applied to cotton using various dyeing times, temperatures, dye bath concentrations and mordants. -

United States Patent Office Patented Sept
3,343,905 United States Patent Office Patented Sept. 26, 1967 2 their washing fastness improved to acceptable levels. 3,343,905 After-treatments of this sort are objectionable from the NYLON DYES Standpoint of both time and cost, but, until now have been James F. Feeman, Wyomissing, Pa., assignor to Crompton the best means available to the dyer for the production of & Knowles Corporation, Worcester, Mass., a corporar Washfast heavy dyeings on nylon. tion of Massacialsetts Other classes of dyes applicable to nylon are similarly No Drawing. Fied Oct. 20, 1966, Ser. No. 587,987 unsuited to production of washfast heavy dyeings. Dis 3 Claims. (C. 8-26) perse dyes are notoriously poor to washing fastness, even This application discloses and claims subject matter though they produce level dyeings with ease and of satis contained in my copending applications, Ser. No. 328,051, O factory depth. Their washfastness is not improved by filed Dec. 4, 1963, now Patent No. 3,305,539 and Ser. after-treatments to the same extent that is found with the No. 378,460, filed June 26, 1964, now abandoned. neutral dyeing acid dyes. Mordant dyes may be metallized This invention relates to dyes for nylon fibers, to a (notably chromed) on nylon by after-treatments with method of dyeing nylon fibers and to the products pro metal salts in acidic baths yielding dyeings of excellent duced by such process. fastness to washing, but the dyeing procedure is very dif Dyeing of nylon in heavy depths of shade with a high ficult to control to the degree necessary to enable the order of fastness to washing is an important technical matching of shades. -

Jacquard Products Currently Trending
Jacquard Products Currently Trending Check out these creative trends and all the ways Jacquard’s products support them! Trends Jacquard Products Notes Metallic Colors • Piñata Alcohol Ink Metallic, iridescent, and pearlescent colors are perpetually • Lumiere popular and currently trending. Jacquard is the #1 choice for • Lumiere 3D these types of colors, no matter what the application. Melony Bradley • Jacquard Screen Printing Ink • Pearl Ex Powdered Pigments • Airbrush Color @julieolsonart chasenfratz.com Yarn Dyeing • Acid Dye (for wool) Needle arts are perennially in vogue, and DIY yarn dyeing is currently trending. Jacquard offers the most vibrant colors available for wool yarn and roving. Dye single color skeins or create a whole rainbow out of yarn! Tara Warburton from wearemakers.com @ahandmadejourney Rock Painting • Neopaque Not just for pet rocks anymore! Decorative painted rocks • Lumiere are wonderfully personalized gifts, adorn indoor and outdoor gardens, and make charming conversation pieces. Lumiere and Neopaque are the most permanent options on the kalescobar @ market for rock painting. Cosplay • Acid Dye Acid Dye is ideal for using with nylon (including nylon wigs) and spandex materials. • Lumiere Lumiere can make virtually any surface look metallic, great • Neopaque for faux armor, weaponry, etc. @miezcosplay Neopaque is permanent on wide range of materials, including plastics and metal. @winged.studio • Basic Dye Basic Dye is excellent for acrylic fibers, wigs, and faux fur. • iDye Poly iDye Poly is great for all of the above, plus epoxy resin, @mckrakenworkshop polycarbonate plastic, and polyester wigs. Polymer Clay • Pearl Ex Powdered Pigments Use Pearl Ex Pigments to achieve metallic and pearlescent clay pieces, before or after baking them to cure. -
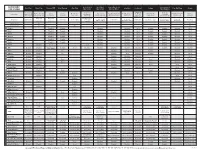
Jacquard Dye Guide
JACQUARD Green Label Red Label Super Fast Acid Concentrated Acid Dye Basic Dye Procion MX iDye Natural iDye Poly SolarFast Cochineal Indigo Dye-Na-Flow Piñata DYE GUIDE Silk Colors Silk Colors Dye for Wool Vinyl Sulphon Natural acid Exceptionally Ready-to-use Maximally Brighter colors than Superior Best for The only option Concentrated Specially formulated for Fixed by exposure to dye. Mordant Natural vat dye Fluid fabric paint that All-surface Product Notes vibrant color for formulation for concentrated any other dye class permanence over-dyeing for polyester versatile liquid color difficult protein fibers sunlight (UV) required for (“blue jean dye”) simulates a dye alcohol ink protein fibers silk painting versatile liquid dye cellulosic fibers Room Temp Room Temp Sets with steam at Sets with steam at Room Temp, Sets with steam at Room Temp. Temp / Heat 180°F / 80°C to to 180°F / 80°C 212°F / 100°C 212°F / 100°C 180°F / 80°C 180°F / 80°C Room Temp or higher Room Temp 212°F or 100°C above 40°F / 4.44°C 212°F Or 100°C Fix by ironing. 160°F / 70°C 32°F / 0°C or fixative Soluble In Alcohol Yes Yes Soluble In Acetone Yes Yes Yes Cotton Excellent Excellent Excellent Excellent Good Excellent Excellent Excellent Good Linen Excellent Excellent Excellent Excellent Good Excellent Excellent Excellent Good Rayon Excellent Excellent Excellent Excellent Good Excellent Excellent Excellent Good Viscose Excellent Excellent Excellent Excellent Good Excellent Excellent Excellent Good Bamboo Excellent Excellent Excellent Excellent Good Excellent Excellent