Cyanogera Bromide and Cyanogen. by AUGUSTUSEDWARD DIXON and JOHNTAYLOR
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent Office Patented Jan
3,071,593 United States Patent Office Patented Jan. 1, 1963 2 3,071,593 O PREPARATION OF AELKENE SULFES Paul F. Warner, Philips, Tex., assignor to Philips Petroleum Company, a corporation of Delaware wherein each R is selected from the group consisting of No Drawing. Filed July 27, 1959, Ser. No. 829,518 5 hydrogen, alkyl, aryl, alkaryl, aralkyl and cycloalkyl 8 Claims. (C. 260-327) groups having 1 to 8 carbon atoms, the combined R groups having up to 12 carbon atoms. Examples of Suit This invention relates to a method of preparing alkene able compounds are ethylene oxide, propylene oxide, iso sulfides. Another aspect relates to a method of convert butylene oxide, a-amylene oxide, styrene oxide, isopropyl ing an alkene oxide to the corresponding sulfide at rela O ethylene oxide, methylethylethylene oxide, 3-phenyl-1, tively high yields without refrigeration. 2-propylene oxide, (3-methylphenyl) ethylene oxide, By the term "alkene sulfide' as used in this specifica cyclohexylethylene oxide, 1-phenyl-3,4-epoxyhexane, and tion and in the claims, I mean to include not only un the like. substituted alkene sulfides such as ethylene sulfide, propyl The salts of thiocyanic acid which I prefer to use are ene sulfide, isobutylene sulfide, and the like, but also 5 the salts of the alkali metals or ammonium. I especially hydrocarbon-substituted alkene sulfides such as styrene prefer ammonium thiocyanate, sodium thiocyanate, and oxide, and in general all compounds conforming to the potassium thiocyanate. These compounds can be reacted formula with ethylene oxide in a cycloparaffin diluent to produce 20 substantial yields of ethylene sulfide and with little or S no polymer formation. -

Recent Advances in Cyanamide Chemistry: Synthesis and Applications
molecules Review Recent Advances in Cyanamide Chemistry: Synthesis and Applications M. R. Ranga Prabhath, Luke Williams, Shreesha V. Bhat and Pallavi Sharma * School of Chemistry, Joseph Banks Laboratories, University of Lincoln, Lincoln LN6 7DL, UK; [email protected] (M.R.R.P.); [email protected] (L.W.); [email protected] (S.V.B.) * Correspondence: [email protected]; Tel.: +44-015-2288-6885 Academic Editor: Margaret A. Brimble Received: 9 March 2017; Accepted: 7 April 2017; Published: 12 April 2017 Abstract: The application of alkyl and aryl substituted cyanamides in synthetic chemistry has diversified multi-fold in recent years. In this review, we discuss recent advances (since 2012) in the chemistry of cyanamides and detail their application in cycloaddition chemistry, aminocyanation reactions, as well as electrophilic cyanide-transfer agents and their unique radical and coordination chemistry. Keywords: cyanamide; synthesis; aminocyanation; cycloaddition; electrophilic cyanation; radical reaction; coordination chemistry 1. Introduction Cyanamide enjoys a rich chemical history, which can be traced to its unique and chemically promiscuous nitrogen-carbon-nitrogen (NCN) connectivity. The chemistry of the nitrile-substituted amino-group of the ‘cyanamide-moiety’ is dominated by an unusual duality of a nucleophilic sp3-amino nitrogen and an electrophilic nitrile unit. The reported use of unsubstituted cyanamide (NH2CN) and metal cyanamides (MNCN, where M = metal) date back as far as the late 19th century, where the likes of calcium cyanamide (CaNCN) was used as a fertilizer, and later as source of ammonia and nitric acid, which fueled the industrial production of metal cyanamides. In contrast, the reported use of the corresponding substituted organic cyanamides (RNHCN or RR’NCN) gathered pace only in more recent years. -

House Fly Attractants and Arrestante: Screening of Chemicals Possessing Cyanide, Thiocyanate, Or Isothiocyanate Radicals
House Fly Attractants and Arrestante: Screening of Chemicals Possessing Cyanide, Thiocyanate, or Isothiocyanate Radicals Agriculture Handbook No. 403 Agricultural Research Service UNITED STATES DEPARTMENT OF AGRICULTURE Contents Page Methods 1 Results and discussion 3 Thiocyanic acid esters 8 Straight-chain nitriles 10 Propionitrile derivatives 10 Conclusions 24 Summary 25 Literature cited 26 This publication reports research involving pesticides. It does not contain recommendations for their use, nor does it imply that the uses discussed here have been registered. All uses of pesticides must be registered by appropriate State and Federal agencies before they can be recommended. CAUTION: Pesticides can be injurious to humans, domestic animals, desirable plants, and fish or other wildlife—if they are not handled or applied properly. Use all pesticides selectively and carefully. Follow recommended practices for the disposal of surplus pesticides and pesticide containers. ¿/áepé4áaUÁí^a¡eé —' ■ -"" TMK LABIL Mention of a proprietary product in this publication does not constitute a guarantee or warranty by the U.S. Department of Agriculture over other products not mentioned. Washington, D.C. Issued July 1971 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 - Price 25 cents House Fly Attractants and Arrestants: Screening of Chemicals Possessing Cyanide, Thiocyanate, or Isothiocyanate Radicals BY M. S. MAYER, Entomology Research Division, Agricultural Research Service ^ Few chemicals possessing cyanide (-CN), thio- cyanate was slightly attractive to Musca domes- eyanate (-SCN), or isothiocyanate (~NCS) radi- tica, but it was considered to be one of the better cals have been tested as attractants for the house repellents for Phormia regina (Meigen). -

Material Safety Data Sheet
Material Safety Data Sheet Silver Thiocyanate (Powder), 99% (Titr.) ACC# 55179 Section 1 - Chemical Product and Company Identification MSDS Name: Silver Thiocyanate (Powder), 99% (Titr.) Catalog Numbers: AC419390000, AC419390250 Synonyms: None. Company Identification: Acros Organics N.V. One Reagent Lane Fair Lawn, NJ 07410 For information in North America, call: 800-ACROS-01 For emergencies in the US, call CHEMTREC: 800-424-9300 Section 2 - Composition, Information on Ingredients CAS# Chemical Name Percent EINECS/ELINCS 1701-93-5 Thiocyanic Acid, Silver(1+) Salt 99 216-934-9 Section 3 - Hazards Identification EMERGENCY OVERVIEW Appearance: white powder. Caution! May cause eye and skin irritation. May cause respiratory and digestive tract irritation. Light sensitive. Moisture sensitive. The toxicological properties of this material have not been fully investigated. Target Organs: None known. Potential Health Effects Eye: May cause eye irritation. The toxicological properties of this material have not been fully investigated. Skin: May cause skin irritation. The toxicological properties of this material have not been fully investigated. Ingestion: May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated. Ingestion of soluble silver salts may cause argyria, characterized by permanent blue-gray pigmentation of the skin, mucous membranes, and eyes. Ingestion of silver compounds may cause abdominal pain, rigidity, convulsions and shock. Inhalation: May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Chronic: No information found. Section 4 - First Aid Measures Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. -

Thiocyanate Reagent Product Code R-1305K Recommended Use Use As Directed by Manufacturer for Purposes Directly Related to Water Testing
SAFETY DATA SHEET 1. Identification Product identifier Thiocyanate Reagent Product code R-1305K Recommended use Use as directed by manufacturer for purposes directly related to water testing. Recommended restrictions None known Manufacturer/Importer/Supplier/Distributor information Manufacturer Company name Anderson Chemical Company Address 325 South Davis Avenue Litchfield, MN 55355 United States Telephone (320) 693-2477 Monday─Friday, 8:00 a.m.–4:30 p.m. Website www.accomn.com E-mail [email protected] Emergency phone number (800) 424-9300 2. Hazard(s) identification Physical hazards This mixture does not meet the classification criteria according to OSHA HazCom 2012. Health hazards This mixture does not meet the classification criteria according to OSHA HazCom 2012. Environmental hazards Not currently regulated by OSHA. For additional information, refer to section 12 of the SDS. Label elements None required Signal word None required Hazard statement None required Precautionary statement Prevention None required Response None required Storage None required Disposal None required Hazard(s) not otherwise classified None Supplemental information None 3. Composition/information on ingredients Mixtures Chemical name Common name and synonyms CAS number % Deionized water Dihydrogen oxide 7732-18-5 95–99 Potassium thiocyanate Thiocyanic acid, potassium salt 333-20-0 0.1–5 4. First-aid measures Inhalation Move to fresh air. Give oxygen or artificial respiration if needed. Get medical attention immediately. Skin contact Immediately wash skin with soap and water. If symptoms persist or in all cases of concern, seek medical advice. Material name: Thiocyanate Reagent; R-1305K SDS U.S. Page 1 of 8 Eye contact Immediately flush eyes with plenty of water for at least 20 minutes. -

Ammonium Thiocyanate
AMMONIUM THIOCYANATE PRODUCT IDENTIFICATION CAS NO. 1762-95-4 EINECS NO. 217-175-6 FORMULA NH 4SCN MOL WT. 76.12 H.S. CODE 2838.00 TOXICITY Oral Rat LD50: 750 mg/kg SYNONYMS Thiocyanic acid, ammonium salt; Amthio; ammonium sulfocanide; ammonium sulphocyanide; ammonium rhodanide; ammonium sulphocyanate; Ammonium Rhodonide; Amthio; Ammonium sulfocyanate; DERIVATION CLASSIFICATION PHYSICAL AND CHEMICAL PROPERTIES PHYSICAL STATE Colorless, deliquescent crystals MELTING POINT 150 C BOILING POINT SPECIFIC GRAVITY 1.31 SOLUBILITY IN WATER Soluble SOLVENT SOLUBILITY Soluble : acetone, alcohol, and ammonia pH 4.5-6.0 (5% sol) VAPOR DENSITY AUTOIGNITION NFPA RATINGS Health: 2 Flammability: 1 Reactivity: 1 REFRACTIVE INDEX FLASH POINT 190 C STABILITY Stable under ordinary conditions APPLICATIONS Cyanic acid (the isomer of fulminic acid) is an unstable (explosive), poisonous, volatile, clear liquid with the structure of H-O-C¡ÕN (the oxoacid formed from the pseudohalogen cyanide), which is readily converted to cyamelide and fulminic acid. There is another isomeric cyanic acid with the structure of H-N=C=O, called isocyanic acid. Cyanate group (and isocyanate group) can react with itself. Cyanuric acid (also called pyrolithic acid), white monoclinic crystal with the structure of [HOC(NCOH) 2N], is the trimer of cyanic acid. The trimer of isocyanic acid is called biuret. • Cyanic acid: H-N=C=O or H-O-C¡ÕN • Fulminic acid: (H-C=N-O) or H-C¡ÕN-O • Isocyanic acid: H-N=C=O • Cyanuric acid: HOC(NCOH) 2N • Biuret: (NH 2)CO) 2 NH Cyanic acid hydrolyses to ammonia and carbon dioxide in water. The salts and esters of cyanic acid are cyanates. -

Reverse-Phase High-Performance Liquid Chromatography of Hydrophobic Proteins and Fragments Thereof’
ANALYTICAL BIOCHEMISTRY 131,99-107 (1983) Reverse-Phase High-Performance Liquid Chromatography of Hydrophobic Proteins and Fragments Thereof’ GEORGE E.TARR*'~ ANDJOHN W.CRABB-~~ *Department of Biological Chemistry, University of Michigan, Ann Arbor, Michigan 48109, and lDepartment of Ophthalmology, University of Washington, Seattle, Washington 98195 Received January 31, 1983 Reverse-phase high-performance liquid chromatography (HPLC) resolution and recovery of cytochrome P-450 and bovine rhodopsin, both integral membrane proteins, and large peptides derived from P-450 LMI were enhanced by utilizing ternary solvents. Surprisingly, most test materials eluted later in the gradient when using mixtures of acetonitrile and propanol in the mobile phase compared to using either solvent alone. Of the supports tested, the best recovery of hydrophobic cytochrome P-450 LMI was experienced on the less retentive CN-bonded phase. Two alternate solvents for HPLC of polypeptides are proposed: (1) 0.02-o. 1 M hexafluoroacetone/ NH3, pH 7.2 for highly acidic peptides; and (2) 6 M formic acid/O.13 M trimethylamine, pH 1.5, vs 4 M formic acid/O.09 M trimethylamine in propanol for relatively insoluble peptides. Anomalous side reactions between formic acid and peptides can cause HPLC peak broadening, increased retention, and decreased resolution. These deleterious effects are thought to be due in part to formyl esterilication of serine and threonine residues and appear to be reversible by aminoethanol treatment. High-performance liquid chromatography, tides (3-7) and hydrophobic, membrane-as- particularly reverse-phase chromatography, sociated polypeptides (8- 14), methods for such has supplanted the more classical methods of fractionations are not yet well established. -

CSAT Top-Screen Questions OMB PRA # 1670-0007 Expires: 5/31/2011
CSAT Top-Screen Questions January 2009 Version 2.8 CSAT Top-Screen Questions OMB PRA # 1670-0007 Expires: 5/31/2011 Change Log .........................................................................................................3 CVI Authorizing Statements...............................................................................4 General ................................................................................................................6 Facility Description.................................................................................................................... 7 Facility Regulatory Mandates ................................................................................................... 7 EPA RMP Facility Identifier....................................................................................................... 9 Refinery Capacity....................................................................................................................... 9 Refinery Market Share ............................................................................................................. 10 Airport Fuels Supplier ............................................................................................................. 11 Military Installation Supplier................................................................................................... 11 Liquefied Natural Gas (LNG) Capacity................................................................................... 12 Liquefied Natural Gas Exclusion -

Cyanogen Bromide; CASRN 506-68-3
Integrated Risk Information System (IRIS) U.S. Environmental Protection Agency Chemical Assessment Summary National Center for Environmental Assessment Cyanogen bromide; CASRN 506-68-3 Human health assessment information on a chemical substance is included in the IRIS database only after a comprehensive review of toxicity data, as outlined in the IRIS assessment development process. Sections I (Health Hazard Assessments for Noncarcinogenic Effects) and II (Carcinogenicity Assessment for Lifetime Exposure) present the conclusions that were reached during the assessment development process. Supporting information and explanations of the methods used to derive the values given in IRIS are provided in the guidance documents located on the IRIS website. STATUS OF DATA FOR Cyanogen bromide File First On-Line 09/26/1988 Category (section) Assessment Available? Last Revised Oral RfD (I.A.) yes 09/26/1988* Inhalation RfC (I.B.) not evaluated Carcinogenicity Assessment (II.) not evaluated *A comprehensive review of toxicological studies was completed (2004) - please see section I.A.6 for more information. I. Chronic Health Hazard Assessments for Noncarcinogenic Effects I.A. Reference Dose for Chronic Oral Exposure (RfD) Substance Name — Cyanogen bromide CASRN — 506-68-3 Last Revised — 09/26/1988 The oral Reference Dose (RfD) is based on the assumption that thresholds exist for certain toxic effects such as cellular necrosis. It is expressed in units of mg/kg-day. In general, the RfD is an estimate (with uncertainty spanning perhaps an order of magnitude) of a daily exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk 1 Integrated Risk Information System (IRIS) U.S. -
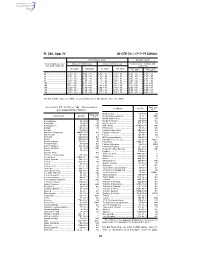
40 CFR Ch. I (7–1–19 Edition)
Pt. 266, App. IV 40 CFR Ch. I (7–1–19 Edition) Noncomplex terrain Complex terrain Terrain-adjusted effec- Values for urban areas Values for rural areas Values for use in urban and tive stack height (m) rural areas Cl2 (g/hr) HCl (g/hr) Cl2 (g/hr) HCl (g/hr) Cl2 (g/hr) HCl (g/hr) 80 .................................. 2.9E + 03 ....... 5.0E + 04 ....... 1.0E + 04 ....... 1.8E + 05 ....... 1.3E + 03 ....... 2.3E + 04 85 .................................. 3.3E + 03 ....... 5.8E + 04 ....... 1.2E + 04 ....... 2.2E + 05 ....... 1.4E + 03 ....... 2.5E + 04 90 .................................. 3.7E + 03 ....... 6.6E + 04 ....... 1.4E + 04 ....... 2.5E + 05 ....... 1.6E + 03 ....... 2.9E + 04 95 .................................. 4.2E + 03 ....... 7.4E + 04 ....... 1.7E + 04 ....... 3.0E + 05 ....... 1.8E + 03 ....... 3.2E + 04 100 ................................ 4.8E + 03 ....... 8.4E + 04 ....... 2.1E + 04 ....... 3.6E + 05 ....... 2.0E + 03 ....... 3.5E + 04 105 ................................ 5.3E + 03 ....... 9.2E + 04 ....... 2.4E + 04 ....... 4.3E + 05 ....... 2.3E + 03 ....... 3.9E + 04 110 ................................ 6.2E + 03 ....... 1.1E + 05 ....... 2.9E + 04 ....... 5.1E + 05 ....... 2.5E + 03 ....... 4.5E + 04 115 ................................ 7.2E + 03 ....... 1.3E + 05 ....... 3.5E + 04 ....... 6.1E + 05 ....... 2.8E + 03 ....... 5.0E + 04 120 ................................ 8.2E + 03 ....... 1.4E + 05 ....... 4.1E + 04 ....... 7.2E + 05 ....... 3.2E + 03 ....... 5.6E + 04 [56 FR 32691, July 17, 1991, as amended at 71 FR 40277, July 14, 2006] APPENDIX IV TO PART 266—REFERENCE RAC (ug/ Constituent CAS No. 3 AIR CONCENTRATIONS* m ) Methoxychlor ............................... 72–43–5 50 Constituent CAS No. -

United States Patent Office 2,395,455
Patented Feb. 26, 1946 2,395,455 UNITED STATES PATENT OFFICE 2,395,455 . DHYDRONoRPoLYCYCLOPENTADIENYL SOBOCYANATES Herman A. Bruson, Philadelphia, Pa., assignor to The Resinous Products & Chemical Company, Philadelphia, Pa., a corporation of Delaware No Drawing. Application January 13, 1945, Seria No. 57239 9 Claims (CL 260-454) This invention relates to addition-rearrange endomethylene group and possess two double. ment products of nascent thiocyanic acid (HSCN) bonds, only one of which, however, responds to and polycyclopentadienes having two double the reaction with thiocyanic acid even when in bonds and one to four endomethylene cycles per excess. Individual, relatively pure, polycyclo molecule. It further relates to a method for the 5 pentadienes may be used or mixtures of these preparation of these new products. hydrocarbons. In accordance with the disclosure of the pres The reaction of polycyclopentadienes and nas ent application, which is a continuation-in-part cent thiocyanic acid takes place particularly rap of application Serial No. 476,646, filed February idly in the presence of water-at temperatures 20, 1943, thiocyanic acid is reacted in the pres 0 of about 60° C. to about 110° C., although some ence of water with polycyclopentadienes having what lower and higher temperatures may be used. the formula: In the preferred temperature range little, if any, polymerization of thiocyanic acid occurs. This acid is generated in situ from a thiocyanate salt 5 and a strong, non-Oxidizing acid which is strong er than thiocyanic acid. The reaction between polycyclopentadiene and thiocyanic acid is carried out by mixing a salt of thiocyanic acid, water, and polycyclopentadi so ene, heating the mixture to a reacting tempera ture, preferably 60° C. -
![Gas Phase Detection and Rotational Spectroscopy of Ethynethiol, HCCSH Arxiv:1811.12798V1 [Astro-Ph.GA] 30 Nov 2018](https://docslib.b-cdn.net/cover/0923/gas-phase-detection-and-rotational-spectroscopy-of-ethynethiol-hccsh-arxiv-1811-12798v1-astro-ph-ga-30-nov-2018-2950923.webp)
Gas Phase Detection and Rotational Spectroscopy of Ethynethiol, HCCSH Arxiv:1811.12798V1 [Astro-Ph.GA] 30 Nov 2018
Gas phase detection and rotational spectroscopy of ethynethiol, HCCSH Kin Long Kelvin Lee,∗,y Marie-Aline Martin-Drumel,z Valerio Lattanzi,{ Brett A. McGuire,x,y Paola Caselli,{ and Michael C. McCarthyy yHarvard-Smithsonian Center for Astrophysics, 60 Garden Street, Cambridge MA 02138, USA zInstitut des Sciences Mol´eculaires d'Orsay, CNRS, Univ Paris Sud, Universit´e Paris-Saclay, Orsay, France {The Center for Astrochemical Studies, Max-Planck-Institut f¨urextraterrestrische Physik, Garching, Germany xNAASC, National Radio Astronomy Observatory, Charlottesville VA 22903, USA E-mail: [email protected] arXiv:1811.12798v1 [astro-ph.GA] 30 Nov 2018 1 Abstract We report the gas-phase detection and spectroscopic characterization of ethynethiol (HCCSH), a metastable isomer of thioketene (H2C2S) using a combination of Fourier- transform microwave and submillimeter-wave spectroscopies. Several a-type transitions of the normal species were initially detected below 40 GHz using a supersonic expansion- electrical discharge source, and subsequent measurement of higher-frequency, b-type lines using double resonance provided accurate predictions in the submillimeter region. With these, searches using a millimeter-wave absorption spectrometer equipped with a radio frequency discharge source were conducted in the range 280 { 660 GHz, ultimately r r yielding nearly 100 transitions up to R0(36) and Q0(68). From the combined data set, all three rotational constants and centrifugal distortion terms up to the sextic order were determined to high accuracy, providing a reliable set of frequency predictions to the lower end of the THz band. Isotopic substitution has enabled both a determination of the molecular structure of HCCSH and, by inference, its formation pathway in our nozzle discharge source via the bimolecular radical-radical recombination reaction SH+ C2H, which is calculated to be highly exothermic (-477 kJ/mol) using the HEAT345(Q) thermochemical scheme.