Pelagic Larval Duration Links Life History Traits and Species Persistence in Darters (Percidae: Etheostomatinae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Docket No. FWS–HQ–ES–2019–0009; FF09E21000 FXES11190900000 167]](https://docslib.b-cdn.net/cover/5635/docket-no-fws-hq-es-2019-0009-ff09e21000-fxes11190900000-167-75635.webp)
Docket No. FWS–HQ–ES–2019–0009; FF09E21000 FXES11190900000 167]
This document is scheduled to be published in the Federal Register on 10/10/2019 and available online at https://federalregister.gov/d/2019-21478, and on govinfo.gov DEPARTMENT OF THE INTERIOR Fish and Wildlife Service 50 CFR Part 17 [Docket No. FWS–HQ–ES–2019–0009; FF09E21000 FXES11190900000 167] Endangered and Threatened Wildlife and Plants; Review of Domestic and Foreign Species That Are Candidates for Listing as Endangered or Threatened; Annual Notification of Findings on Resubmitted Petitions; Annual Description of Progress on Listing Actions AGENCY: Fish and Wildlife Service, Interior. ACTION: Notice of review. SUMMARY: In this candidate notice of review (CNOR), we, the U.S. Fish and Wildlife Service (Service), present an updated list of plant and animal species that we regard as candidates for or have proposed for addition to the Lists of Endangered and Threatened Wildlife and Plants under the Endangered Species Act of 1973, as amended. Identification of candidate species can assist environmental planning efforts by providing advance notice of potential listings, and by allowing landowners and resource managers to alleviate threats and thereby possibly remove the need to list species as endangered or threatened. Even if we subsequently list a candidate species, the early notice provided here could result in more options for species management and recovery by prompting earlier candidate conservation measures to alleviate threats to the species. This document also includes our findings on resubmitted petitions and describes our 1 progress in revising the Lists of Endangered and Threatened Wildlife and Plants (Lists) during the period October 1, 2016, through September 30, 2018. -

Status Review and Surveys for Frecklebelly Madtom, Noturus Munitus
Noturus munitus, Buttahatchee R., Lowndes Co., AL Final Report: Status review and surveys for Frecklebelly Madtom, Noturus munitus. David A. Neely, Ph. D. Tennessee Aquarium Conservation Institute 175 Baylor School Rd Chattanooga, TN 37405 Background: The frecklebelly madtom, Noturus munitus, was described by Suttkus & Taylor (1965) based largely on specimens from the Pearl River in Louisiana and Mississippi. At that time, the only other known populations occupied the Cahaba and Upper Tombigbee rivers in Alabama and Mississippi. Populations were subsequently discovered in the Alabama, Etowah, and Conasauga rivers (Bryant et. al., 1979). The disjunct distribution displayed by the species prompted an examination of morphological and genetic differentiation between populations of frecklebelly madtom. Between 1997-2001 I gathered morphological, meristic, and mtDNA sequence data on frecklebelly madtoms from across their range. Preliminary morphological data suggested that while there was considerable morphological variation across the range, the populations in the Coosa River drainage above the Fall Line were the most distinctive population and were diagnosable as a distinct form. I presented a talk on this at the Association of Southeastern Biologists meeting in 1998, and despite the lack of a formal description, the published abstract referring to a "Coosa Madtom" made it into the public 1 eye (Neely et al 1998, Boschung and Mayden 2004). This has resulted in substantial confusion over conservation priorities and the status of this form. The subsequent (and also unpublished) mitochondrial DNA data set, however, suggested that populations were moderately differentiated, shared no haplotypes and were related to one another in the following pattern: Pearl[Tombigbee[Cahaba+Coosa]]. -

A Thesis Entitled Molecular, Morphological, and Biogeographic Resolution of Cryptic Taxa in the Greenside Darter Etheostoma Blen
A Thesis Entitled Molecular, morphological, and biogeographic resolution of cryptic taxa in the Greenside Darter Etheostoma blennioides complex By Amanda E. Haponski Submitted as partial fulfillment of the requirements for The Master of Science Degree in Biology (Ecology-track) ____________________________ Advisor: Dr. Carol A. Stepien ____________________________ Committee Member: Dr. Timothy G. Fisher ____________________________ Committee Member: Dr. Johan F. Gottgens ____________________________ College of Graduate Studies The University of Toledo December 2007 Copyright © 2007 This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Molecular, morphological, and biogeographic resolution of cryptic taxa in the Greenside Darter Etheostoma blennioides complex Amanda E. Haponski Submitted as partial fulfillment of the requirements for The Master of Science Degree in Biology (Ecology-track) The University of Toledo December 2007 DNA sequencing has led to the resolution of many cryptic taxa, which are especially prevalent in the North American darter fishes (Family Percidae). The Greenside Darter Etheostoma blennioides commonly occurs in the lower Great Lakes region, where two putative subspecies, the eastern “Allegheny” type E. b. blennioides and the western “Prairie” type E. b. pholidotum , overlap. The objective of this study was to test the systematic identity and genetic divergence distinguishing the two subspecies in areas of sympatry and allopatry in comparison to other subspecies and close relatives. DNA sequences from the mtDNA cytochrome b gene and control region and the nuclear S7 intron 1 comprising a total of 1,497 bp were compared from 294 individuals across 18 locations, including the Lake Erie basin and the Allegheny, Meramec, Obey, Ohio, Rockcastle, Susquehanna, and Wabash River systems. -

2020 Mississippi Bird EA
ENVIRONMENTAL ASSESSMENT Managing Damage and Threats of Damage Caused by Birds in the State of Mississippi Prepared by United States Department of Agriculture Animal and Plant Health Inspection Service Wildlife Services In Cooperation with: The Tennessee Valley Authority January 2020 i EXECUTIVE SUMMARY Wildlife is an important public resource that can provide economic, recreational, emotional, and esthetic benefits to many people. However, wildlife can cause damage to agricultural resources, natural resources, property, and threaten human safety. When people experience damage caused by wildlife or when wildlife threatens to cause damage, people may seek assistance from other entities. The United States Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services (WS) program is the lead federal agency responsible for managing conflicts between people and wildlife. Therefore, people experiencing damage or threats of damage associated with wildlife could seek assistance from WS. In Mississippi, WS has and continues to receive requests for assistance to reduce and prevent damage associated with several bird species. The National Environmental Policy Act (NEPA) requires federal agencies to incorporate environmental planning into federal agency actions and decision-making processes. Therefore, if WS provided assistance by conducting activities to manage damage caused by bird species, those activities would be a federal action requiring compliance with the NEPA. The NEPA requires federal agencies to have available -

C:\Fish\Eastern Sand Darter Sa.Wpd
EASTERN SAND DARTER STATUS ASSESSMENT Prepared by: David Grandmaison and Joseph Mayasich Natural Resources Research Institute University of Minnesota 5013 Miller Trunk Highway Duluth, MN 55811-1442 and David Etnier Ecology and Evolutionary Biology University of Tennessee 569 Dabney Hall Knoxville, TN 37996-1610 Prepared for: U.S. Fish and Wildlife Service Region 3 1 Federal Drive Fort Snelling, MN 55111 January 2004 NRRI Technical Report No. NRRI/TR-2003/40 DISCLAIMER This document is a compilation of biological data and a description of past, present, and likely future threats to the eastern sand darter, Ammocrypta pellucida (Agassiz). It does not represent a decision by the U.S. Fish and Wildlife Service (Service) on whether this taxon should be designated as a candidate species for listing as threatened or endangered under the Federal Endangered Species Act. That decision will be made by the Service after reviewing this document; other relevant biological and threat data not included herein; and all relevant laws, regulations, and policies. The result of the decision will be posted on the Service's Region 3 Web site (refer to: http://midwest.fws.gov/eco_serv/endangrd/lists/concern.html). If designated as a candidate species, the taxon will subsequently be added to the Service's candidate species list that is periodically published in the Federal Register and posted on the World Wide Web (refer to: http://endangered.fws.gov/wildlife.html). Even if the taxon does not warrant candidate status it should benefit from the conservation recommendations that are contained in this document. ii TABLE OF CONTENTS DISCLAIMER................................................................... -

Lessons from the Tellico Dam Controversy
Volume 43 Issue 4 Fall 2003 Fall 2003 Public Goods Provision: Lessons from the Tellico Dam Controversy Louis P. Cain Brooks A. Kaiser Recommended Citation Louis P. Cain & Brooks A. Kaiser, Public Goods Provision: Lessons from the Tellico Dam Controversy, 43 Nat. Resources J. 979 (2003). Available at: https://digitalrepository.unm.edu/nrj/vol43/iss4/4 This Article is brought to you for free and open access by the Law Journals at UNM Digital Repository. It has been accepted for inclusion in Natural Resources Journal by an authorized editor of UNM Digital Repository. For more information, please contact [email protected], [email protected], [email protected]. LOUIS P. CAIN & BROOKS A. KAISER* Public Goods Provision: Lessons from the Tellico Dam Controversy ABSTRACT Although absent from the initial Endangered Species Act (ESA) of 1973, evidence of economic considerationsfirst appeared in the 1978 amendments. The only controversial vote concerning the ESA was the one to exempt the Tellico Dam (1978). Although the dam was a local project with little expected net benefit, this article argues that broader economic considerations mattered. Working from public choice models for congressional voting decisions, a limited dependent variable regression analysis indicates the economic variables with the most explanatory power for this environmental decision are college education, poverty, the designation of critical habitat within a district, the number of endangered species in the state, dollars the state received due to earlier ESA funding, and the percentage of the district that is federal land. Comparisons with aggregated environmental votes in the same year highlight the intensity of economic considerations in the Tellico case. -
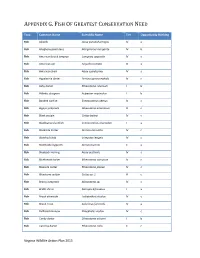
Fish of Greatest Conservation Need
APPENDIX G. FISH OF GREATEST CONSERVATION NEED Taxa Common Name Scientific Name Tier Opportunity Ranking Fish Alewife Alosa pseudoharengus IV a Fish Allegheny pearl dace Margariscus margarita IV b Fish American brook lamprey Lampetra appendix IV c Fish American eel Anguilla rostrata III a Fish American shad Alosa sapidissima IV a Fish Appalachia darter Percina gymnocephala IV c Fish Ashy darter Etheostoma cinereum I b Fish Atlantic sturgeon Acipenser oxyrinchus I b Fish Banded sunfish Enneacanthus obesus IV c Fish Bigeye jumprock Moxostoma ariommum III c Fish Black sculpin Cottus baileyi IV c Fish Blackbanded sunfish Enneacanthus chaetodon I a Fish Blackside darter Percina maculata IV c Fish Blotched chub Erimystax insignis IV c Fish Blotchside logperch Percina burtoni II a Fish Blueback Herring Alosa aestivalis IV a Fish Bluebreast darter Etheostoma camurum IV c Fish Blueside darter Etheostoma jessiae IV c Fish Bluestone sculpin Cottus sp. 1 III c Fish Brassy Jumprock Moxostoma sp. IV c Fish Bridle shiner Notropis bifrenatus I a Fish Brook silverside Labidesthes sicculus IV c Fish Brook Trout Salvelinus fontinalis IV a Fish Bullhead minnow Pimephales vigilax IV c Fish Candy darter Etheostoma osburni I b Fish Carolina darter Etheostoma collis II c Virginia Wildlife Action Plan 2015 APPENDIX G. FISH OF GREATEST CONSERVATION NEED Fish Carolina fantail darter Etheostoma brevispinum IV c Fish Channel darter Percina copelandi III c Fish Clinch dace Chrosomus sp. cf. saylori I a Fish Clinch sculpin Cottus sp. 4 III c Fish Dusky darter Percina sciera IV c Fish Duskytail darter Etheostoma percnurum I a Fish Emerald shiner Notropis atherinoides IV c Fish Fatlips minnow Phenacobius crassilabrum II c Fish Freshwater drum Aplodinotus grunniens III c Fish Golden Darter Etheostoma denoncourti II b Fish Greenfin darter Etheostoma chlorobranchium I b Fish Highback chub Hybopsis hypsinotus IV c Fish Highfin Shiner Notropis altipinnis IV c Fish Holston sculpin Cottus sp. -

Information on the NCWRC's Scientific Council of Fishes Rare
A Summary of the 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes in North Carolina Submitted by Bryn H. Tracy North Carolina Division of Water Resources North Carolina Department of Environment and Natural Resources Raleigh, NC On behalf of the NCWRC’s Scientific Council of Fishes November 01, 2014 Bigeye Jumprock, Scartomyzon (Moxostoma) ariommum, State Threatened Photograph by Noel Burkhead and Robert Jenkins, courtesy of the Virginia Division of Game and Inland Fisheries and the Southeastern Fishes Council (http://www.sefishescouncil.org/). Table of Contents Page Introduction......................................................................................................................................... 3 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes In North Carolina ........... 4 Summaries from the 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes in North Carolina .......................................................................................................................... 12 Recent Activities of NCWRC’s Scientific Council of Fishes .................................................. 13 North Carolina’s Imperiled Fish Fauna, Part I, Ohio Lamprey .............................................. 14 North Carolina’s Imperiled Fish Fauna, Part II, “Atlantic” Highfin Carpsucker ...................... 17 North Carolina’s Imperiled Fish Fauna, Part III, Tennessee Darter ...................................... 20 North Carolina’s Imperiled Fish Fauna, Part -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Stability, Persistence and Habitat Associations of the Pearl Darter Percina Aurora in the Pascagoula River System, Southeastern USA
Vol. 36: 99–109, 2018 ENDANGERED SPECIES RESEARCH Published June 13 https://doi.org/10.3354/esr00897 Endang Species Res OPENPEN ACCESSCCESS Stability, persistence and habitat associations of the pearl darter Percina aurora in the Pascagoula River System, southeastern USA Scott R. Clark1,4,*, William T. Slack2, Brian R. Kreiser1, Jacob F. Schaefer1, Mark A. Dugo3 1Department of Biological Sciences, The University of Southern Mississippi, Hattiesburg, Mississippi 39406, USA 2US Army Engineer Research and Development Center, Environmental Laboratory EEA, Vicksburg, Mississippi 39180, USA 3Mississippi Valley State University, Department of Natural Sciences and Environmental Health, Itta Bena, Mississippi 38941, USA 4Present address: Department of Biology and Museum of Southwestern Biology, University of New Mexico, Albuquerque, New Mexico 87131, USA ABSTRACT: The southeastern United States represents one of the richest collections of aquatic biodiversity worldwide; however, many of these taxa are under an increasing threat of imperil- ment, local extirpation, or extinction. The pearl darter Percina aurora is a small-bodied freshwater fish endemic to the Pearl and Pascagoula river systems of Mississippi and Louisiana (USA). The last collected specimen from the Pearl River drainage was taken in 1973, and it now appears that populations in this system are likely extirpated. This reduced the historical range of this species by approximately 50%, ultimately resulting in federal protection under the US Endangered Species Act in 2017. To better understand the current distribution and general biology of extant popula- tions, we analyzed data collected from a series of surveys conducted in the Pascagoula River drainage from 2000 to 2016. Pearl darters were captured at relatively low abundance (2.4 ± 4.0 ind. -

A Synopsis of the Biology and Life History of Ruffe
J. Great Lakes Res. 24(2): 170-1 85 Internat. Assoc. Great Lakes Res., 1998 A Synopsis of the Biology and Life History of Ruffe Derek H. Ogle* Northland College Mathematics Department Ashland, Wisconsin 54806 ABSTRACT. The ruffe (Gymnocephalus cernuus), a Percid native to Europe and Asia, has recently been introduced in North America and new areas of Europe. A synopsis of the biology and life history of ruffe suggests a great deal of variability exists in these traits. Morphological characters vary across large geographical scales, within certain water bodies, and between sexes. Ruffe can tolerate a wide variety of conditions including fresh and brackish waters, lacustrine and lotic systems, depths of 0.25 to 85 m, montane and submontane areas, and oligotrophic to eutrophic waters. Age and size at maturity dif- fer according to temperature and levels of mortality. Ruffe spawn on a variety ofsubstrates, for extended periods of time. In some populations, individual ruffe may spawn more than once per year. Growth of ruffe is affected by sex, morphotype, water type, intraspecific density, and food supply. Ruffe feed on a wide variety of foods, although adult ruffe feed predominantly on chironomid larvae. Interactions (i.e., competition and predation) with other species appear to vary considerably between system. INDEX WORDS: Ruffe, review, taxonomy, reproduction, diet, parasite, predation. INTRODUCTION DISTRIBUTION This is a review of the existing literature on Ruffe are native to all of Europe except for along ruffe, providing a synopsis of its biology and life the Mediterranean Sea, western France, Spain, Por- history. A review of the existing literature is tugal, Norway, northern Finland, Ireland, and Scot- needed at this time because the ruffe, which is na- land (Collette and Banarescu 1977, Lelek 1987). -

Underwater Observation and Habitat Utilization of Three Rare Darters
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 5-2010 Underwater observation and habitat utilization of three rare darters (Etheostoma cinereum, Percina burtoni, and Percina williamsi) in the Little River, Blount County, Tennessee Robert Trenton Jett University of Tennessee - Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Natural Resources and Conservation Commons Recommended Citation Jett, Robert Trenton, "Underwater observation and habitat utilization of three rare darters (Etheostoma cinereum, Percina burtoni, and Percina williamsi) in the Little River, Blount County, Tennessee. " Master's Thesis, University of Tennessee, 2010. https://trace.tennessee.edu/utk_gradthes/636 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Robert Trenton Jett entitled "Underwater observation and habitat utilization of three rare darters (Etheostoma cinereum, Percina burtoni, and Percina williamsi) in the Little River, Blount County, Tennessee." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Wildlife and Fisheries Science. James L. Wilson, Major Professor We have read this thesis and recommend its acceptance: David A. Etnier, Jason G.