Jemds.Com Original Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Route No. 1, Driver-Mr. Suresha K, 7204134544, Seat-56
PESIT Bangalore South Campus – College Bus Route Chart Session: July 2016 – Jun 2017 Route No. 1, Driver-Mr. Suresha K, 7204134544, Seat-56 Pick up Points Pick up Time Annual Fees Banaswadi Main Road-HP Petrol Bunk 06:30 RS Palya Main Road 6:35 Kamanahalli Cross 6:35 Rs. 20,000/- Uttam sagar 6:40 Dodda Banaswadi 6:40 Ramamurthynagar Jn. 6:40 Kasturinagar Ring Road 6:40 Tin Factory 6:45 KR Puram railway Station 6:50 B Narayanapura Ring Road Jn. 6:55 Rs. 18,000/- Mahadevpura Ring Road Jn. 6:55 Dodda Nakundi Ring Road Jn. 7:00 Kartiknagar Ring Road Jn. 7:05 Sarjapura Fire Station / Total Mall 7:15 Rs. 16,000/- Kai Kondarahalli-Jail Road 7:20 Kasavanahalli / Amrita University 7:20 Naganathapura 7:25 Rs. 14,000/- PESIT Bangalore South Campus 7:45 Route No. 2, Driver-Mr. Jagadeesha K, Mobile-9538019116, Seat-40 Pick up Points Pick up Time Annual Fees Ganganagar / CBI Office 6:30 am Mekhri Circle 6:30 am Fun World / Nandidurga Road / 6:40am Cole’s Park Rs. 20,000/- Halsoor Lake (Ganapathy Temple) 6:45am Philps 6:45am Old Madras Road 6:45am CMH Hospital(80feet Road) 6:50am Thippasandra (Bata Show Room) 6:50am Indira Nagar 100 feet Road 6:55am Rs. 18,000/- New Horizon School 6:55am Domlur Flyover 7:05am Ejipura Signal Koramangala - Sony World 7:15am Rs. 16,000/- Madivala - Silk Board 7:25am Bommanahalli - Rs. 14,000/- PESIT Bangalore South Campus 7:45am Route No. 3, Driver Mr. Palaksha NB, Mobile-9900258408 Seat-40 Pick up Points Pick up Time Annual Fees KFC - Indiriranagar 6:40 am Jeevanbimanagar 6:40 am HAL Airport(Arrival Gate) 6:50 am Rs. -

Unclaimed Dividend 2011
THE KARUR VYSYA BANK LIMITED, REGD. CENTRAL OFFICE: ERODE ROAD, KARUR 639002 [CIN No: L65110TN1916PLC001295] List of Unpaid dividend 2011‐12 transferred to IEPF Sl No Folio/ Demat ID SHARES STATUS PREFIX INITLS NAME AD1 AD2 AD3 AD4 PINCOD NETDIV DWNO 1 A00015 35 1 ALAGARSAMI CHETTIAR A S C/O G S A MOHAN DOSS 173/10 BIG BAZAR STREET CUMBUM-626 516 626516 490.00 1216730 2 A00054 420 1 ANASUYA K R 25 RAJAJI STREET KARUR 639001 5,880.00 1200477 3 A00057 134 1 ANBU SUBBIAH R 4 GANDHI NAGAR IST CROSS KARUR 639001 1,876.00 1200170 4 A00122 1142 1 ARJUNA BAI 68 BAZAAR STREET KEMPANAICKENPALAYAM VIA D G PUDUR ERODE R M S 638503 15,988.00 1200133 5 A00144 33 1 ALAMELU N 33 SOUTH CAR STREET PALANI 624 601 ANNA DISTRICT 624601 462.00 1200478 6 A00263 112 1 ARUMUGAM K 1 DAMASCUS ROAD NEW FAIRLANDS SALEM-636 016 636016 1,568.00 1218678 7 A00329 604 1 ALAMELU R 80 CAR STREET KARUR 639001 8,456.00 1201862 8 A00344 11 1 AMSA SEKHARAN S 58 I CROSS THILLAIPURAM NAMAKKAL 637001 154.00 1219168 9 A00416 9 1 ANNAPOORANI S W/O SURESH KUMAR, OFFICER THE KARUR VYSYA BANK LTD 45-46, CAR STREET SALEM 636001 126.00 1217742 10 A00428 100 1 ANUSUYA S 275 CHINNA KADAI STREET, SALEM 636001 1,400.00 1217743 11 A00435 33 1 ANUSUYA P 14 PULIYUR SECOND LANE KODAMBAKKAM MADRAS 600 024 600024 462.00 1200479 12 A00454 22 1 ARUMUGAM T 77 K V B NAGAR KARUR 2 639002 308.00 1200172 13 A00457 56 1 ARUNA B NO.9/11, M.M.INDUSTRIAL ROAD 7TH BLOCK, JAYANAGAR WEST YEDIYUR BANGALORE 560082 784.00 1210846 14 A00463 22 1 ASAITHAMBI K 22-C RATHINAM STREET KARUR-639001 639001 308.00 1201866 -

(Expired) SRI VENKATESH IYER DN 28, 2Nd Cross, Nehrunagar, Bangalore
DM 8 (Expired) DM 9 (Expired) LM 14 SRI VENKATESH IYER D.N. SRI SRINIVASAN. V SRI KRISHNA. V 28, 2nd Cross, Nehrunagar, 1023, Aruna, IV Block, Rajajinagar, # 112, Flat No. 1, ‗Vijaya Apartments‘ Bangalore - 560 020. Bangalore - 560 010 7th Cross, 9th Main, R.M.V. Extn., Bangalore - 560 060 LM 17 DM 18 LM 19 SRI KRISHNAMURTHY. G.S. SRI SAMPANGIRAMAIAH. M. N SRI SRINIVASA IYER. S #1275, Govindarajnagar, No. 69, South Cross Road, No. 46/5, 18th Cross, N.S.Palya, Bangalore - 560079 Basavanagudi, Bangalore - 560004 B.T.M. 2nd Stage, Ph No.: 23351450 (R), Ph No.: 65363536 Bangalore - 560 076 23366990 (Off) LM 21 LM 26 (Expired) LM 27 (Expired) SRI NAGARAJA IYER. C.S. SRI SURYANARAYANAN. V SRI SRINIVASA IYER. S.V. #384/12, 6th Main Road, 14th # 112, Flat No. 1, ‗Vijaya Aprts‘, 7th Yelagondahalli P.O. (Via) Cross,Lakkasandra Extension, Cross, 9th Main, R.M.V. Extn., D.S.M. 563 127 Wilson Garden Bangalore - 560 060 Bangalore - 560 030 LM 28 (Expired) LM 29 LM 30 SRI VENKATESHAN. T.S. SRI SHANKARA IYER. K.R. SRI MURTHY K.R.S # 67, 10th ‗B‘ Main Road,1st No. 14, ‗Hemalatha, 3rd Cross, 6/1, 5th Main, Chamarajpet, Block, Jayanagar, Govipuram Extn., Bangalore - 560 018 (Expired) Bangalore - 560 011, Bangalore – 560 019.(Expired) LM 32 (Expired) LM 33 LM 34 SRI SESHADRI. V.A. SRI THYAGARAJA IYER. D. SRI NAGABHUSHANA. S 10, 2nd Cross, 4, 19th Cross, Malleshwaram, 4, 'Amrit' Upper Pipeline Cross, Shankarapuram, Bangalore - 560 055 Seshadripuram, Bangalore - 560 Bangalore - 560 004 020 LM 35 LM 36 LM 37 SRI ASHOK. -
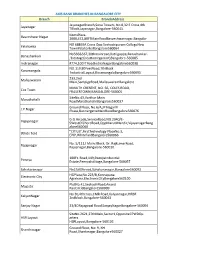
Designated Bank Branches List for Payment
AXIS BANK BRANCHES IN BANGALORE CITY Branch BranchAddress Jayanagar JayanagarBranch,Sona Towers, No 8,32 E Cross,4th TBlock,Jayanagar,Bangalore-560041. KeersPlaza- Basveshwar Nagar 2000,472,80FTMainRoadBasveshwarnagar,Bangalor e560079 NO 688 IIIA Cross Opp.Seshadripuram CollegeNew Yelahanka TownYelahankaBangalore560064 No566&567,30thmainroad,Katriguppe,Banashankari Banashankari ,3rdstage(nexttomegamart)Bangalore-560085 Indiranagar #774,100FTRoadIndiraNagarBangalore560038 NO.119,80FeetRoad,7thBlock Koramangala IndustrialLayout,KoramangalaBangalore560095 233,2nd Malleswaram Main,SampigeRoad,MalleswaramBangalore 560003 MINUTH CRESENT, NO. 56, COLES ROAD, Cox Town FRASERTOWN,BANGALORE-560005 SiteNo.43,Varthur Main Marathahalli RoadMarathahalliIBangalore560037 Ground Floor, No.6/A,JP NagarIII J.P.Nagar Phase,BannergattaMainRoadBangalore560076 G.G.Arcade,ServiceRoad,NO.2940/E- Vijayanagar 5WestOfChordRoad,OppMarutiMandir,VijayanagarBang alore560040 "CITIUS",FirstTechnology PlaceNo.3, White field EPIP,WhitefieldBangalore560066 No. 5/111,I Main,IBlock, Dr. RajkumarRoad, Rajajinagar Rajajinagar,Bangalore-560010. 100Ft. Road,149,PeenyaIndustrial Peenya Estate,PeenyaIstStage,Bangalore-560057 Sahakaranagar No19,60ftroad,Sahakaranagar,Bangalore560092 HGPlaza,No.223/B,Konnapana Electronic City Agrahara,ElectronicCityBangalore560100 PlotNo.41,SeshadriRoad,Anand Majestic RaoCircleBangalore560009 No 30,4thcross,CMR Road,Kalyannagar,HRBR KalyanNagar 2ndblock,Bangalore-560043 Sanjay Nagar 23/4DRajagopal Road,SanjayNagarBangalore-560094 SiteNo.2621,27thMain,Sector1,OppositeCPWDQu -

CSR Karnataka Conference
BETHE INSPIRATION Organized by: Rotary International District 3190 Event managed by: Rotary Bangalore Jeevanbima Nagar Rotary Karnataka CSR Conference 2019 ROTARY : SERVICE ABOVE SELF February 15, 2019 VENUE : Sir M. V. Auditorium, Federation of Karnataka Chambers of Commerce and Industry (FKCCI), K.G.Road, Bengaluru, Karnataka Rotary District 3190 is organizing its Second Annual Karnataka CSR conference on 15th Feb 2019.This conference is to celebrate the contribution made by various stakeholders towards community service in Karnataka for socio-economic development of this State. GOLD SPONSOR AWARDS KNOWLEDGE PARTNER SDGs OUTREACH PARTNER Tech Care for All a social impact company www.tc4a.com We at Rotary International District 3190 The discussions at the rst CSR always have community service at the core conference ranged in topics such as of our DNA and would like to recognize all education, skills development, health the corporate citizens who are doing their & sanitation, green Karnataka, and mite towards this objective. Rotary District rural development. The varied panels 3190 is celebrating its second Annual that covered these topics included Karnataka CSR event on 15th Feb 2019; This C-level representatives and CSR conference is to celebrate the contribution leaders from various foundations made by various stakeholder towards and companies such as : community service in various veicals that you have implemented in Karnataka for socio-economic development of this State. The world we belong to is a much beer place than yesterday-Thanks to Corporate Citizens like you! Ÿ Are we giving back more than what we take from Society? Ÿ Are we moving towards Strategic CSR and sustainability in our activities to create economic output and wealth? Ÿ Would we be leaving the world a beer place for the next generation that what we bequeathed from our previous generation? Ÿ Are we aligning ourselves to SDG 2030 and making signicant contribution towards improving HDI These would be the overarching questions we will ruminate upon when we meet on Feb 15, 2019. -

BANGALORE CORPORATION VOTER LIST.Xlsx
Sl. Reg No. Name And address 1 69 Reg No. 69 Shri Hemanth V Swami No. 502, 'Arla Vilas', 6th A Main RMV 2nd Stage, Bangalore-560 094 Bangalore North Taluk 2 77 Reg No.77 Shri Patil Ravikanth Shankarppa No. 139/1, 5th Main Chamarajpet, Bangalore -560018 Bangalore North Taluk 3 80 Reg No. 80 Shri Anil Vishwanth Rikke Flat No. 1, Block- 01, White House R T Nagar, Bangalore -560032 Bangalore North Taluk 4 Reg No.133 Reg No.133 Shri Chi Mallikarjunaswami S/o Jayanna No. 2822, Narasinhswami Nilaya Meenakshinagar, Kamakshipalya, Bangalore -560079, Bangalore North Taluk 5 194 Reg No. 194 Shri P Mahadeva S/o Puttaiah No. 433, 434, South India Tours, Alankar Plaza, KG Road, Bangalore -09 Bangalore North Taluk 6 26 Reg No. 26 Shri S C Shivananjappa S/o S.P.Chikkaveerashetty No. 8, Suhas, 7th 'B' Main,3rd Stage 4th Block, Basaveshwarnagar, Bangalore -560079Bangalore North Taluk 7 29 Reg No. 29 Shri G P Ravishankar No.S-99, KEB Colony 3rd Stage, 3rd Cross Basaveshwarnagar, Bangalore - 560079 Bangalore North Taluk 8 30 Reg No. 30 Dr. Sunil C. Shirola No.14, 1st Main, 2nd A Cross KHB Colony, 1st Stage, Basaveshwarnagar Bangalore-560079, Bangalore North Taluk 9 31 Reg No. 31 Shri G. Anand No.212, I 'G' Cross, 3rd Stage 4th Block, Basaveshwaranagar Bangalore-560 079 Bangalore North Taluk 10 32 Reg No. 32 Shri A N Devaraj No. 73, Basaveshwar Road Millers Road, Bangalore -560052 11 249 Reg No. 249 Shri T. V. Nagaraj Chamundeshwar Electricals, No. 818 5th Main, 6th Cross, MC Layout, Vijayanagar, Bangalore -560040 Bangalore North Taluk 12 665 Reg No. -

Incorporating Equity in Public Transport Planning: the Case Of
Curtin University Sustainability Policy (CUSP) Institute Incorporating Equity in Public Transport Planning: The case of Bengaluru Jyothi Chava A thesis by hybrid publication submitted in fulfilment of the requirement for the Degree of Doctor of Philosophy (PhD) of Curtin University September 2016 DECLARATION To the best of my knowledge and belief this thesis contains no material previously published by any other person except where due acknowledgment has been made. This thesis contains no material that has been accepted for the award of any other degree or diploma in any university. Human Ethics: The research presented and reported in this thesis was conducted in accordance with the National Health and Medical Research Council National Statement on Ethical Conduct in Human Research (2007) – updated March 2014. The proposed research study received human research ethics approval from the Curtin University Human Research Ethics Committee (EC00262), Approval Number # HURGS-04-14. Signature: Date: 12/09/16. i ABSTRACT Public transport (PT) and its associated developments are emerging as sustainable urban transport solutions. However, the rapidly increasing investments on them are not yielding equitable benefits for all. To address these inequity concerns, the study proposes a methodology to evaluate and incorporate equity related aspects into PT planning at the station area and network levels, and demonstrates the methods in Bengaluru, India as a case study. The equity solutions for station area level planning are illustrated in Yeshwanthpur industrial area (in Bengaluru’s suburbs) as the primary case study. At the station area level, the study developed a series of methods to: assess which income groups are being excluded from transit oriented developments (TOD); identify gentrification in TODs; evaluate the probability of replacement, in future, of the poor from TODs; and assess the implication of such social exclusion on PT ridership, through the development of a new model. -

As Per the List Annexed
HCE 623/2006 High Court of Karnataka, Bengaluru, Dated 15 th June 2018. VIVA – VOCE INTIMATION LETTER Viva – voce to the eligible candidates for selection to the post of Law Clerk - cum - Research Assistant on the establishment of High Court of Karnataka as called vide Notification No. HCE 623/2006 dated 05.03.218 is scheduled to be held on dates as mentioned hereunder at 04.30.pm in the premises of the High Court of Karnataka, Bengaluru – 560001. Therefore, the candidates as per Annexure – ‘A’ annexed to this intimation letter are hereby required to be present on the said dates at 3.00 PM for verification of your original documents at Recruitment Branch, High Court of Karnataka, Principal Bench, Bengaluru – 560001. Further, you are required to produce all the relevant original certificates regarding proof of date of birth, qualification, Enrolment Certificate issued by the concerned Bar Council, achievement in co-curricular activities, etc, at the time of viva - voce. If the original documents relied upon by you are not produced at the time of viva-voce, the candidate will be disqualified to attend the viva – voce. BY ORDER, Sd /- (B. JAYANTHA KUMAR) REGISTRAR (RECRUITMENT) To, AS PER THE LIST ANNEXED 1 of 7 HCE 623/2006 Viva-Voce on Tuesday, the 26th June 2018 Sl. Sl. Address Address No. No. No. No. Appln. Appln. Appln. MR. ARPIT KUMAR MALLICK MS. PRIYANKA CHAVAN S/o ANIL KUMAR MALLICK D/o CHANDRAKANT, 1 1 No. 12 U.A., Jawahar Nagar, 8 9 H.No-1172, At Post-Hebbal, Near Malkaganj Chowk, Hukkeri (T), Delhi- 110007 Belagavi (D)- 591221 MR. -

GML March 15.Cdr
March 2015 Issue: 9 38th ROTARY DISTRICT-3190 CONFERENCE 21st & 22nd Feb 2015 uddipana The Rotary District 3190 Conference, held at the Nimhans The RI Rep. was welcomed in typical Mysore style at 8.30 am, with a Auditorium, Bangalore, on the 21st and 22nd February 2015, Mysore Peta and Shawl, at the conference venue, with traditional under the stewardship of District Governor Rtn. Manjunath Shetty, drums and folk dancers leading the procession, with RI Dir. Elect was a memorable Conference, unique in several aspects, and Manoj Desai joining the dignitaries. The traditional lamp was lit in the thoroughly appreciated by the 1800 strong Rotarians and families foyer, with all the luminaries present, marking the inauguration of the who attended in full strength, on both days. Conference. The Conference Calendar began with a Lunch, hosted at the Century Club, on the 20th, to welcome the RI representative PDG Anil Agarwal and Ann Seema, all District Officers, Invitees, Club Presidents, and Patrons of the Conference, which set the tone and the mood for what was to follow, over the next 2 days. Rtn. Anil TELL US WHY YOU JOINED ROTARY Agarwal addressing the informal gathering, expressed his happiness to be amongst us, and was keen on participating in the 2 The Rotarian Magazine is working on a story about ways to take advantage day event. A meeting of the College of Governors, discussing and of your Rotary membership. Tell us why you joined Rotary -- and why you debating important issues, and a well-represented Press stayed -- and you may see yourself quoted in the August issue. -

Lvo-45A Rc Details-123
ÀЦÑÍ´ ÌБÐÔÜ ƒ¸°º¦ÐÔÀÐÔ 2005¤Ð »Ðõ‘Фб ‘Ðà 4 (1)(Š) ªÁ.vÉ.¸À.D, ¸ÀܽÃAiÀÄ ªÀiË®åªÀ¢üðvÀ vÉjUÉ PÀbÉÃj-045(C¥ÀgÀ), ¨ÉAUÀ¼ÀÆgÀÄ. Details of RC Records in the office of ACCT, LVO-045(Addl.), Bangalore as on 30-09-2011. ‘ЮгзРÀЗÓþ ‘ЮгÐÀйÐÔî ‘Ð®Ð³ÐÀйÐÔî ‘Ð®Ð³ÐÀйÐÔî ‘Ð®Ð³Ð ‘ЮгР¹ÑÆÐ ÁÈЦÐÔÁÈЦÐÔÁÈЦÐÔ ‘ЮгР‘ЮгРƒ³Ðô ‘ЮгРºÀÐþÌв٠‘ЮгР‘ЮгР‘ÐõÀÐÔ ·Ñ“ÄÑ´–ÐÎÐ ‘ЮгР‘Фб ƒ¿°ÄÙÓ”ÑæÐÔ ƒ¿°ÄÙÓ”ÑæÐÔ‘ÙÜ ƒ¿°ÄÙÓ”ÑæРƒ¿°ÄÙÓ”Ñ Ã¦ÐÔ·Ð ¹ÑÆЖÙÖÏÊÐÃÔ »Ð¯Ë·Ð »Ñõ¤Ð¿°Ë·Ð –ÙÖÏË·Ð ÁÄÙÓÀѧ ¹ÑÆЖÙÖÏË· ÊДÙôÊДÙôÊДÙô ÊДÙôÊДÙôÊДÙô Š ¿ Ë ·ÐÅö ‘ÐÎÐÔÍË·Ð ‘ÐÎÐÔÍ˷Р˽ñ¸ Ô·ÐÅö »Ð®Ù·Ð ƒ¸°‘ѧ ÌÙÊФÐÔ „·ÙÓÇË·Ð ƒ¸°‘ѧ ÀÐÈÐþÀÐÈÐþÀÐÈÐþ ¸¹Ñ‘и¹Ñ‘и¹Ñ‘Ð ÀÐÈÐþÀÐÈÐþÀÐÈÐþ Ð ¸¹Ñ‘ÐÐ ¸¹Ñ‘Ð ¯ … ¸¹Ñ‘и¹Ñ‘и¹Ñ‘Ð ÌÙÊФÐÔÌÙÊФÐÔÌÙÊФÐÔ ¸¹Ñ‘и¹Ñ‘и¹Ñ‘Ð ƒ¸°‘ѧ ÌÙÊФÐÔ ÌÙÊФÐÔÌÙÊФÐÔÌÙÊФÐÔ ¤Ñô‘ý ¹Ð¤Ñô‘ý ¹Ð½®ÐÄý ¹Ð. ÀÐÈÐþÀÐÈÐþÀÐÈÐþ 1 2 3(1) 3(2) 4 5 6 7 8 9 10 11a 11b 11c 12 13 14 15 GROUP P SHOP.NO.8, 1ST FLOOR, NO.761/1, OUTER RING ROAD, P & P INTERIOR 1 29580877931 ABOVE AVARAW, SERVICE 12/01/2010 Running file A 10/13/2011 Arul Mary 10/13/2011 D.K. -

PESIT Bangalore South Campus – College Bus Route Chart Session: July 2018 – Jun 2019
PESIT Bangalore South Campus – College Bus Route Chart Session: July 2018 – Jun 2019 Route No. 1, Driver: Mr. Suresha K, 7204134544, Seats - 55 Pick up Points Pick up Time Annual Fees Banaswadi Main Road - HP Petrol Bunk 06:20 RS Palya Main Road 6:25 Kammanahalli Cross 6:25 Rs. 24,000/- Uttam Sagar 6:30 Horamau Signal 6:30 Ramamurthynagar Jn. 6:40 Kasturinagar Ring Road 6:40 Tin Factory 6:45 KR Puram Railway Station 6:50 B Narayanapura Ring Road Jn. 6:50 Rs. 22,000/- Mahadevpura Ring Road Jn. 6:55 Doddanekkundi Ring Road Jn. 7:00 Kartiknagar Ring Road Jn. 7:05 Sarjapura Fire Station / Total Mall 7:10 Rs. 18,000/- Haraluru Road 7:15 Naganathapura 7:20 Central Jail Road / Hosa Road Jn. 7:25 Rs. 16,000/- PESIT Bangalore South Campus 7:45 Route No. 2, Driver: Mr. Jagadeesha K., Mobile: 9538019116, Seats - 50 Pick up Points Pick up Time Annual Fees Ganganagar / CBI Office 6:20 am Mekhri Circle 6:20 am Fun World / Nandidurga Road / Cole’s Park 6:25am Rs. 24,000/- Halsoor Lake (Ganapathy Temple) 6:35am Phillips 6:35am Old Madras Road 6:40am KFC Indiranagar 6:45am 6:45am Indira Nagar 100 feet Road 6:50am Rs. 22,000/- New Horizon School 6:50am Domlur Flyover 6:55am Ejipura Signal Koramangala 7:00am Sony World 7:00am Rs. 18,000/- Madivala 7:10am Silk Board 7:15am Bommanahalli 7:20am Rs. 16,000/- PESIT Bangalore South Campus 7:45am Route No. 3, Driver: Mr. Palaksha, Mobile: 9900258408, Seats - 40 Pick up Points Pick up Time Annual Fees KFC - Indiriranagar 6:30 am Jeevanbimanagar 6:35 am HAL Airport(Arrival Gate) 6:40 am Rs. -

In the High Court of Karnataka at Bengaluru
1 IN THE HIGH COURT OF KARNATAKA AT BENGALURU DATED THIS THE 30 TH DAY OF MARCH, 2021 BEFORE THE HON' BLE MR.JUSTICE R. DEVDAS WRIT PETITION NO.15481/2020(EDN-RES) C/W WRIT PETITION NOs.1143/2021, 745/2021, 830/2021, 858/2021 (EDN-RES) IN W.P. NO.15481/2020 BETWEEN: 1. DR. SADHWINI. M H, AGED 27 YEARS, D/O M P HARISH R/O #10141, ASPIRE 10, SOBHA APARTMENTS, NAGASANDRA, BENGALURU– 560073 2. DR. RICHA SINGH D/O BIJENDER KUMAR AGED 28 YEARS R/O 321#, 8 TH MAIN ROAD, 1 ST BLOCK HRBR LAYOUT, KALYAN NAGAR, BENGALURU 3. DR ASHOKA RAKSHITH AGED 30 YEARS S/O ASHOK KUMAR K.R R/O C/O ARJUN SS. F02/HOUSE #1/1ST FLOOR 2 TEACHERS COLONY, 1ST AVENUE, KORAMANGALA 1ST BLOCK, BENGALURU 560034 4. DR. ANKIT NARANG S/O VED PRAKASH NARANG AGED 30 YRS R/O NARANG CLINIC, FACTORY ROAD, WARD NO.16 NEAR SANJAY PARK PILIBANGA,DT: HANUMANGARH, RAJASTHAN STATE- 335803 5. DR. DHAMSANIA HARDIK JAGDISHBHAI S/O JAGDISHBHAI J DHAMSANIA AGED 31YRS R/O A 37, ALAP AVENUE J K CHOCK,NEAR PUSHKARDHAM, UNIVERSITY ROAD RAJKOT, GUJARAT-360005. 6. DR DEEPTHI RAVISHANKAR D/O B P RAVISHANKAR | AGED ABOUT 28 YEARS R/O : #1415” GURU “ 5 TH STAGE 1ST PHASE, 8TH MAIN 7 TH CROSS, BEML LAYOUT, RR NAGAR, BENGALURU 560098 7. DR. SHIVANSH TRIVEDI S/O GOVIND TRIVEDI AGE : 26 YEARS R/O C/O MENS HOSTEL, J.J.M. MEDICAL COLLEGE, DAVANAGERE 577004 8. DR. SANJAY V C AGED ABOUT 28 YRS S/O CHIDAMBARSHETTY R/O SRI LAKSHMI VENKATESHWARA ANUGRAHA BUILDING, 16-44/1, SINGH LAYOUT.