The Pennsylvania State University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
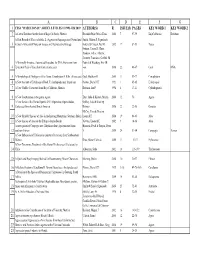
Haseltonia Articles and Authors.Xlsx
ABCDEFG 1 CSSA "HASELTONIA" ARTICLE TITLES #1 1993–#26 2019 AUTHOR(S) R ISSUE(S) PAGES KEY WORD 1 KEY WORD 2 2 A Cactus Database for the State of Baja California, Mexico Resendiz Ruiz, María Elena 2000 7 97-99 BajaCalifornia Database A First Record of Yucca aloifolia L. (Agavaceae/Asparagaceae) Naturalized Smith, Gideon F, Figueiredo, 3 in South Africa with Notes on its uses and Reproductive Biology Estrela & Crouch, Neil R 2012 17 87-93 Yucca Fotinos, Tonya D, Clase, Teodoro, Veloz, Alberto, Jimenez, Francisco, Griffith, M A Minimally Invasive, Automated Procedure for DNA Extraction from Patrick & Wettberg, Eric JB 4 Epidermal Peels of Succulent Cacti (Cactaceae) von 2016 22 46-47 Cacti DNA 5 A Morphological Phylogeny of the Genus Conophytum N.E.Br. (Aizoaceae) Opel, Matthew R 2005 11 53-77 Conophytum 6 A New Account of Echidnopsis Hook. F. (Asclepiadaceae: Stapeliae) Plowes, Darrel CH 1993 1 65-85 Echidnopsis 7 A New Cholla (Cactaceae) from Baja California, Mexico Rebman, Jon P 1998 6 17-21 Cylindropuntia 8 A New Combination in the genus Agave Etter, Julia & Kristen, Martin 2006 12 70 Agave A New Series of the Genus Opuntia Mill. (Opuntieae, Opuntioideae, Oakley, Luis & Kiesling, 9 Cactaceae) from Austral South America Roberto 2016 22 22-30 Opuntia McCoy, Tom & Newton, 10 A New Shrubby Species of Aloe in the Imatong Mountains, Southern Sudan Leonard E 2014 19 64-65 Aloe 11 A New Species of Aloe on the Ethiopia-Sudan Border Newton, Leonard E 2002 9 14-16 Aloe A new species of Ceropegia sect. -

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Sedum Society Newsletter(130) Pp
Open Research Online The Open University’s repository of research publications and other research outputs Kalanchoe arborescens - a Madagascan giant Journal Item How to cite: Walker, Colin (2019). Kalanchoe arborescens - a Madagascan giant. Sedum Society Newsletter(130) pp. 81–84. For guidance on citations see FAQs. c [not recorded] https://creativecommons.org/licenses/by-nc-nd/4.0/ Version: Version of Record Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk NUMBER 130 SEDUM SOCIETY NEWSLETTER JULY 2019 FRONT COVER Roy Mottram kindly supplied: “The Diet” copy of this Japanese herbal which is sharp and crisp (see page 97). “I counted the plates, and this copy is complete with 200 plates, in 8 parts, bound here in 2 vols. I checked for another Sedum but none are Established April 1987, now ending our present, so Maximowicz was basing his 32nd year. S. kagamontanum on this same plate, Subscriptions run from October to the following September. Anyone requesting translating the location as Mt. Kaga and to join after June, unless there is a special citing t.40 incorrectly. The "t.43" plate request, will receive his or her first number is also wrong. It is actually t.33 of Newsletter in October. If you do not the whole work, or Vol.2 t.8. The book is receive your copy by the 10th of April, July or October, or the 15th January, then bound back to front [by Western standards] please write to the editor: Ray as in all Japanese books of the day.” RM. -

CRASSULACEAE 景天科 Jing Tian Ke Fu Kunjun (傅坤俊 Fu Kun-Tsun)1; Hideaki Ohba 2 Herbs, Subshrubs, Or Shrubs
Flora of China 8: 202–268. 2001. CRASSULACEAE 景天科 jing tian ke Fu Kunjun (傅坤俊 Fu Kun-tsun)1; Hideaki Ohba 2 Herbs, subshrubs, or shrubs. Stems mostly fleshy. Leaves alternate, opposite, or verticillate, usually simple; stipules absent; leaf blade entire or slightly incised, rarely lobed or imparipinnate. Inflorescences terminal or axillary, cymose, corymbiform, spiculate, racemose, paniculate, or sometimes reduced to a solitary flower. Flowers usually bisexual, sometimes unisexual in Rhodiola (when plants dioecious or rarely gynodioecious), actinomorphic, (3 or)4– 6(–30)-merous. Sepals almost free or basally connate, persistent. Petals free or connate. Stamens as many as petals in 1 series or 2 × as many in 2 series. Nectar scales at or near base of carpels. Follicles sometimes fewer than sepals, free or basally connate, erect or spreading, membranous or leathery, 1- to many seeded. Seeds small; endosperm scanty or not developed. About 35 genera and over 1500 species: Africa, America, Asia, Europe; 13 genera (two endemic, one introduced) and 233 species (129 endemic, one introduced) in China. Some species of Crassulaceae are cultivated as ornamentals and/or used medicinally. Fu Shu-hsia & Fu Kun-tsun. 1984. Crassulaceae. In: Fu Shu-hsia & Fu Kun-tsun, eds., Fl. Reipubl. Popularis Sin. 34(1): 31–220. 1a. Stamens in 1 series, usually as many as petals; flowers always bisexual. 2a. Leaves always opposite, joined to form a basal sheath; inflorescences axillary, often shorter than subtending leaf; plants not developing enlarged rootstock ................................................................ 1. Tillaea 2b. Leaves alternate, occasionally opposite proximally; inflorescence terminal, often very large; plants sometimes developing enlarged, perennial rootstock. -

Universidad Nacional Autónoma De México Facultad De Estudios
Universidad Nacional Autónoma de México Facultad de Estudios Superiores Zaragoza “LAS CRASULÁCEAS DEL VALLE DEL MEZQUITAL’’ Tesis de licenciatura que para obtener el título de Biólogo presentan: Gabriela de Jesús Espino Ortega Luis Emilio de la Cruz López Área de Botánica Director de tesis: M. en C. Balbina Vázquez Benítez. _____________________________ Marzo 2009 UNAM – Dirección General de Bibliotecas Tesis Digitales Restricciones de uso DERECHOS RESERVADOS © PROHIBIDA SU REPRODUCCIÓN TOTAL O PARCIAL Todo el material contenido en esta tesis esta protegido por la Ley Federal del Derecho de Autor (LFDA) de los Estados Unidos Mexicanos (México). El uso de imágenes, fragmentos de videos, y demás material que sea objeto de protección de los derechos de autor, será exclusivamente para fines educativos e informativos y deberá citar la fuente donde la obtuvo mencionando el autor o autores. Cualquier uso distinto como el lucro, reproducción, edición o modificación, será perseguido y sancionado por el respectivo titular de los Derechos de Autor. Agradecimientos A la UNAM por brindarme la oportunidad de desarrollarme mejor tanto en el sentido humano como en el profesional. Por hacer la diferencia en mi vida. A la FES Zaragoza y a las personas que en ella laboran, por ser mi casa de estudios. Al grupo de sinodales formado por M. en C. Ramiro Ríos Gómez, M. en C. Balbina Vázquez Benítez, M. en C. Efraín Ángeles Cervantes, M. en C. Carlos Castillejos Cruz y Florencia Becerril Cruz, por las observaciones hechas pertinentemente para la mejora de este trabajo, por los comentarios y el tiempo dedicado al mismo. Por su amistad A la Maestra Balbina con quien estaré siempre agradecida porque trabajó con mucho entusiasmo en nuestro trabajo. -

Green Roof Substrate Composition Affects Phedimus Kamtschaticus
HORTSCIENCE 52(2):320–325. 2017. doi: 10.21273/HORTSCI11202-16 ponding. In the early 19th century, green roofs in Berlin did not use engineered media; rather, construction rubble was Green Roof Substrate Composition spread over tar paper roofs and the living systems developed overtime (Kohler and Affects Phedimus kamtschaticus Poll, 2010). Modern GRS composition is largely based on recommendations in the Growth and Substrate Water Content Forschungsgesellschaft Landschaftsent- wicklung Landschaftsbau (FLL), the German landscape industry’s guidelines for the de- under Controlled Environmental sign, planting, and maintenance of green roof systems. The FLL makes recommen- Conditions dations for particle size distribution and 1 organic content as well as specific physical Whitney N. Griffin , Steven M. Cohan, John D. Lea-Cox, properties such as water holding capacity, and Andrew G. Ristvey bulk density, and total porosity (FLL, Department of Plant Sciences and Landscape Architecture, University of 2008). Maryland, Plant Sciences Building, College Park, MD 20740 Beyond the basic FLL recommendations, GRS composition varies internationally and Additional index words. Phedimus, eco roof, living roof, evapotranspiration, plant available regionally, usually due to raw material water, Sedum kamtschaticum availability; however, the FLL recommen- dations have been adopted by municipalities Abstract. Phedimus kamtschaticus (Fischer) were grown in three experimental crushed and public entities around the world and brick-based green roof substrates (GRSs) with increasing organic matter (OM) content Ò applied to green roof components not (10%, 20%, and 40% by volume) and a commercially available blend, Rooflite ,in considered in or by the FLL. North Amer- single-pot replicates in a growth chamber for 6 months. -

A New Combination in Phedimus (Crassulaceae), with Neotypification of Sedum Latiovalifolium
Phytotaxa 278 (3): 294–296 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2016 Magnolia Press Correspondence ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.278.3.10 A new combination in Phedimus (Crassulaceae), with neotypification of Sedum latiovalifolium DONG CHAN SON1, HYUN-JUN KIM1, AE-RA MOON2, CHANG-GEE JANG3 & KAE SUN CHANG1* 1Division of Forest Biodiversity and Herbarium, Korea National Arboretum, Pocheon-si, 11186, Republic of Korea 2Division of Gardens and Education, Korea National Arboretum, Pocheon-si, 11186, Republic of Korea 3Department of Biology Education, Kongju National University, Gongju-si, 32588, Republic of Korea *Corresponding author: [email protected] According to recent molecular studies (‘t Hart 1995, Ham 1995, Ham & ‘t Hart 1998, Gontcharova et al. 2006, Thiede & Eggli 2007, Gontcharova & Gontchaov 2009), the genus Phedimus Rafinesque (1817: 438) was segregated from Sedum Lin- naeus (1753: 430). This taxonomic treatment of Phedimus is also supported by morphological evidences (Ohba et al. 2000, Ohba 2001, Fu et al. 2001). During the preparation of the account of the Crassulaceae for the A Synonymic List of Vascular Plants in Korea, it was decided that Phedimus should be separated from Sedum. Most of correct names in Sedum which is found in Korean peninsula have combinations in Phedimus already. However, Sedum latiovalifolium Y.N.Lee (1992: 8), which is described as a endemic to Korea has not yet been transferred to Phedimus. Meanwhile, regarding taxonomic identity of S. latiovalifolium, ‘t Hart & Bleij (2003) considered that S. latiovali- folium was tentatively treated as synonym of P. -

Drought Resistance Mechanisms of Phedimus Aizoon L. Yuhang Liu1, Zhongqun He1*, Yongdong Xie1,2, Lihong Su1, Ruijie Zhang1, Haixia Wang1, Chunyan Li1 & Shengju Long1
www.nature.com/scientificreports OPEN Drought resistance mechanisms of Phedimus aizoon L. Yuhang Liu1, Zhongqun He1*, Yongdong Xie1,2, Lihong Su1, Ruijie Zhang1, Haixia Wang1, Chunyan Li1 & Shengju Long1 Phedimus aizoon L. is a drought-resistant Chinese herbal medicine and vegetable. However, its drought tolerant limit and the mechanism of drought tolerance are unknown, which restricts the promotion of water-saving cultivation of Phedimus aizoon L. in arid areas. To solve the above problem, we carried out a 30-day-long drought stress experiment in pots that presented diferent soil water contents and were divided into four groups: control check, 75–80% of the maximum water-holding capacity (MWHC); mild drought, 55–60%; moderate drought, 40–45%; and severe drought, 20–25%. The dynamic changes in both plant physiological indexes from 10 to 30 days and leaf anatomical structure on the 30th day of stress were recorded. The results show that Phedimus aizoon L. grew normally under mild drought stress for 30 days, but the growth of the plants became inhibited after 20 days of severe drought and after 30 days of moderate drought. At the same time, Phedimus aizoon L. physiologically responded to cope with drought stress: the growth of the root system accelerated, the waxy layer of the leaves thickened, and the dark reactions of the plants transformed from those of the C3 cycle to CAM. The activity of antioxidant enzymes (SOD, POD and CAT) continuously increased to alleviate the damage caused by drought stress. To ensure the relative stability of the osmotic potential, the contents of osmoregulatory substances such as proline, soluble sugars, soluble protein and trehalose increased correspondingly. -

Kozminska;Al;Wiszniewska
Document downloaded from: http://hdl.handle.net/10251/158945 This paper must be cited as: Kozminska, A.; Al Hassan, M.; Wiszniewska, A.; Hanus-Fajerska, E.; Boscaiu, M.; Vicente, O. (2019). Responses of succulents to drought: Comparative analysis of four Sedum (Crassulaceae) species. Scientia Horticulturae. 243:235-242. https://doi.org/10.1016/j.scienta.2018.08.028 The final publication is available at https://doi.org/10.1016/j.scienta.2018.08.028 Copyright Elsevier Additional Information Responses of succulents to drought: comparative analysis of four Sedum (Crassulaceae) species Aleksandra Kozminska a,b*, Mohamad Al Hassan a,c, Alina Wiszniewska b, Ewa Hanus- Fajerska b , Monica Boscaiu d, Oscar Vicente a a Institute of Plant Molecular and Cellular Biology (IBMCP, UPV-CSIC), Universitat Politècnica de València, Camino de Vera s/n, 46022, Valencia, Spain b Unit of Botany and Plant Physiology of Institute of Plant Biology and Biotechnology, Department of Biotechnology and Agriculture, University of Agriculture in Krakow, 31-425 Krakow, Al. 29 Listopada 54, Poland (permanent address) c Present address: The New Zealand Institute for Plant & Food Research Limited (PFR), Mt Albert Research Centre, Private Bag 92169, Auckland, New Zealand (present address) d Mediterranean Agroforestry Institute (IAM, UPV), Universitat Politècnica de València, Spain, Camino de Vera s/n, 46022, Valencia, Spain *corresponding author e-mail: [email protected] Abstract The increased frequency and intensity of drought periods is becoming a serious thread for agriculture, prompting the identification of crop species and cultivars with enhanced water stress tolerance. Drought responses were studied in four ornamental Sedum species under controlled greenhouse conditions, by withholding watering of the plants for four weeks. -

Naturalized in North America
Phytotaxa 175 (1): 019–028 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2014 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.175.1.2 A taxonomic study of Sedum series Rupestria (Crassulaceae) naturalized in North America LORENZO GALLO1 & PETER F. ZIKA2 1 Strada Val San Martino sup. 194. 10131, Torino, Italy. E-mail: [email protected] 2 WTU Herbarium, Box 355325, University of Washington, Seattle, WA 98195-5325, USA. E-mail: [email protected] Abstract Sedum rupestre L. and its close relatives (Sedum series Rupestria) are native to Europe. Adventive populations in North America were studied both in the field and the herbaria. Our results exclude S. rupestre L. and include recognition of two additional taxa on the continent, which are distinguished with keys and illustrations: Sedum forsterianum documented as a naturalized species in the United States, from Washington, and in British Columbia, Canada and Sedum thartii, naturalized in Colorado, Maine, Ohio, Oregon, Washington and Ontario, Canada. A lectotype is established for S. forsterianum. Key words: Alien, stonecrop, typification Introduction The genus Sedum Linnaeus (1753: 430) is widespread in Europe, Africa, Asia and America and includes roughly 400 to 475 taxa according to the most recent authors (Eggli et al. 1995, ‘t Hart & Bleij 2003, Thiede & Eggli 2007, Ohba 2009). Berger (1930) devised an infrafamilial classification of the Crassulaceae. Within the genus Sedum he recognised “Sektion 17” (Sedum proper, his “Seda genuina”) and, inside it, several “Reihen” or series. The “Reihe” 20 group of Sedum is now known as Sedum series Rupestria (Berger 1930: 456), a monophyletic and well delimited group endemic to the Euro-Mediterranean region, including 17 among species, subspecies and natural hybrids (Hart ‘t 1994, Hart ‘t & Bleij 2003, Gallo 2009, 2012). -

The Genus Stelis Comprises Approximately 105 Species Worldwide, with About 20 to 25% of Them Occurring in the Western Palaearctic and the Middle East
Supplement 18, 144 Seiten ISSN 0250-4413 / ISBN 978-3-925064-71-8 Ansfelden,24.März 2015 The Cuckoo Bees of the Genus Panzer, 1806 in Europe, North Africa and the Middle East A Review and Identification Guide Table of Contents Introduction ............................................................................................................ 4 General Part ............................................................................................................ 7 – Description of the Genus ........................................................................... 7 – Number of Species Described ................................................................... 7 – Species Diversity on Country Level .......................................................... 8 – Abundance of Stelis ................................................................................... 9 – Host Associations ...................................................................................... 9 – Flower Preferences ................................................................................... 15 – Flight Season ............................................................................................ 19 – Sexual Dimorphism .................................................................................. 19 – Geographic Variation ............................................................................... 19 – Taxonomy: The Subgenera of Stelis ........................................................ 20 Coverage and Methodology