And Glaciovolcanic Basaltic Tuffs: Modes of Alteration and Relevance for Mars
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flood Basalts and Glacier Floods—Roadside Geology
u 0 by Robert J. Carson and Kevin R. Pogue WASHINGTON DIVISION OF GEOLOGY AND EARTH RESOURCES Information Circular 90 January 1996 WASHINGTON STATE DEPARTMENTOF Natural Resources Jennifer M. Belcher - Commissioner of Public Lands Kaleen Cottingham - Supervisor FLOOD BASALTS AND GLACIER FLOODS: Roadside Geology of Parts of Walla Walla, Franklin, and Columbia Counties, Washington by Robert J. Carson and Kevin R. Pogue WASHINGTON DIVISION OF GEOLOGY AND EARTH RESOURCES Information Circular 90 January 1996 Kaleen Cottingham - Supervisor Division of Geology and Earth Resources WASHINGTON DEPARTMENT OF NATURAL RESOURCES Jennifer M. Belcher-Commissio11er of Public Lands Kaleeo Cottingham-Supervisor DMSION OF GEOLOGY AND EARTH RESOURCES Raymond Lasmanis-State Geologist J. Eric Schuster-Assistant State Geologist William S. Lingley, Jr.-Assistant State Geologist This report is available from: Publications Washington Department of Natural Resources Division of Geology and Earth Resources P.O. Box 47007 Olympia, WA 98504-7007 Price $ 3.24 Tax (WA residents only) ~ Total $ 3.50 Mail orders must be prepaid: please add $1.00 to each order for postage and handling. Make checks payable to the Department of Natural Resources. Front Cover: Palouse Falls (56 m high) in the canyon of the Palouse River. Printed oo recycled paper Printed io the United States of America Contents 1 General geology of southeastern Washington 1 Magnetic polarity 2 Geologic time 2 Columbia River Basalt Group 2 Tectonic features 5 Quaternary sedimentation 6 Road log 7 Further reading 7 Acknowledgments 8 Part 1 - Walla Walla to Palouse Falls (69.0 miles) 21 Part 2 - Palouse Falls to Lower Monumental Dam (27.0 miles) 26 Part 3 - Lower Monumental Dam to Ice Harbor Dam (38.7 miles) 33 Part 4 - Ice Harbor Dam to Wallula Gap (26.7 mi les) 38 Part 5 - Wallula Gap to Walla Walla (42.0 miles) 44 References cited ILLUSTRATIONS I Figure 1. -

The Hyaloclastite Ridge Formed in the Subglacial 1996 Eruption in Gjfilp, Vatnaj6kull, Iceland: Present Day Shape and Future Preservation
The hyaloclastite ridge formed in the subglacial 1996 eruption in Gjfilp, Vatnaj6kull, Iceland: present day shape and future preservation M. T. GUDMUNDSSON, F. PALSSON, H. BJORNSSON & ~. HOGNADOTTIR Science Institute, University of Iceland, Hofsvallag6tu 53, 107 Reykjavik, Iceland (e-mail: [email protected]) Abstract: In the Gjfilp eruption in 1996, a subglacial hyaloclastite ridge was formed over a volcanic fissure beneath the Vatnaj6kull ice cap in Iceland. The initial ice thickness along the 6 km-long fissure varied from 550 m to 750 m greatest in the northern part but least in the central part where a subaerial crater was active during the eruption. The shape of the subglacial ridge has been mapped, using direct observations of the top of the edifice in 1997, radio echo soundings and gravity surveying. The subglacial edifice is remarkably varied in shape and height. The southern part is low and narrow whereas the central part is the highest, rising 450 m above the pre-eruption bedrock. In the northern part the ridge is only 150-200m high but up to 2kin wide, suggesting that lateral spreading of the erupted material occurred during the latter stages of the eruption. The total volume of erupted material in Gj~tlp was about 0.8 km 3, mainly volcanic glass. The edifice has a volume of about 0.7 km 3 and a volume of 0.07 km 3 was transported with the meltwater from Gj~lp and accumulated in the Grimsv6tn caldera, where the subglacial lake acted as a trap for the sediments. This meltwater-transported material was removed from the southern part of the edifice during the eruption. -

The Pitcairn Hotspot in the South Paci¢C: Distribution and Composition of Submarine Volcanic Sequences
Available online at www.sciencedirect.com R Journal of Volcanology and Geothermal Research 121 (2003) 219^245 www.elsevier.com/locate/jvolgeores The Pitcairn hotspot in the South Paci¢c: distribution and composition of submarine volcanic sequences R. Hekinian a;Ã, J.L. Chemine¤e b, J. Dubois b, P. Sto¡ers a, S. Scott c, C. Guivel d, D. Garbe-Scho«nberg a, C. Devey e, B. Bourdon b, K. Lackschewitz e, G. McMurtry f , E. Le Drezen g a Universita«t Kiel, Institut fu«r Geowissenschaften, OlshausentraMe 40, 24098 Kiel, Germany b Institut de Physique du Globe de Paris, 4 Place Jussieu, 75252 Paris, France c Geology Department, University of Toronto, Toronto, ON, Canada M5S 3B1 d Universite¤ de Nantes, Faculte¤ des Sciences, 2 Rue de la Houssinie're, 92208 Nantes, France e University of Bremen, Geowissenschaften, Postfach 340440, 28334 Bremen, Germany f University of Hawaii, Department of Oceanography, 1000 Pope Road, Honolulu, HI 96822, USA g IFREMER Centre de Brest, Ge¤oscience Marine, 29280 Plouzane¤, France Received 19 March 2002; accepted 30 August 2002 Abstract Multibeam bathymetry and bottom imaging (Simrad EM12D) studies on an area of about 9500 km2 were conducted over the Pitcairn hotspot near 25‡10PS, 129‡ 20PW. In addition, 15 dives with the Nautile submersible enabled us to obtain ground-true observations and to sample volcanic structures on the ancient ocean crust of the Farallon Plate at 3500^4300 m depths. More than 100 submarine volcanoes overprint the ancient crust and are divided according to their size into large ( s 2000 m in height), intermediate (500^2000 m high) and small ( 6 500 m high) edifices. -

Icelandic Hyaloclastite Tuffs Petrophysical Properties, Alteration and Geochemical Mobility
Icelandic Hyaloclastite Tuffs Petrophysical Properties, Alteration and Geochemical Mobility Hjalti Franzson Gudmundur H. Gudfinnsson Julia Frolova Helga M. Helgadóttir Bruce Pauly Anette K. Mortensen Sveinn P. Jakobsson Prepared for National Energy Authority and Reykjavík Energy ÍSOR-2011/064 ICELAND GEOSURVEY Reykjavík: Orkugardur, Grensásvegur 9, 108 Reykjavík, Iceland - Tel.: 528 1500 - Fax: 528 1699 Akureyri: Rangárvellir, P.O. Box 30, 602 Akureyri, Iceland - Tel.: 528 1500 - Fax: 528 1599 [email protected] - www.isor.is Report Project no.: 540105 Icelandic Hyaloclastite Tuffs Petrophysical Properties, Alteration and Geochemical Mobility Hjalti Franzson Gudmundur H. Gudfinnsson Julia Frolova Helga M. Helgadóttir Bruce Pauly Anette K. Mortensen Sveinn P. Jakobsson Prepared for National Energy Authority and Reykjavík Energy ÍSOR-2011/064 December 2011 Key page Report no. Date Distribution ÍSOR-2011/064 December 2011 Open Closed Report name / Main and subheadings Number of copies Icelandic Hyaloclastite Tuffs. Petrophysical Properties, Alteration 7 and Geochemical Mobility Number of pages 103 Authors Project manager Hjalti Franzson, Gudmundur H. Gudfinnsson, Julia Frolova, Hjalti Franzson Helga M. Helgadóttir, Bruce Pauly, Anette K. Mortensen and Sveinn P. Jakobsson Classification of report Project no. 540105 Prepared for National Energy Authority and Reykjavík Energy Cooperators Moscow University, University of California in Davis, Natural History Museum in Iceland Abstract This study attempts to define the properties of hyaloclastite formations which control their petrophysical characteristics during their progressive alteration. It is based on 140 tuffaceous cores from last glaciation to 2–3 m y. The water content shows a progressive increase with alteration to about 12%, which mainly is bound in the smectite and zeolite alteration minerals. -

Durham Research Online
Durham Research Online Deposited in DRO: 07 June 2010 Version of attached le: Published Version Peer-review status of attached le: Peer-reviewed Citation for published item: Jerram, Dougal A. and Single, Richard T. and Hobbs, Richard W. and Nelson, Catherine E (2009) 'Understanding the oshore ood basalt sequence using onshore volcanic facies analogues : an example from the Faroe-Shetland basin.', Geological magazine., 146 (3). pp. 353-367. Further information on publisher's website: http://dx.doi.org/10.1017/S0016756809005974 Publisher's copyright statement: c Copyright Cambridge University Press 2009. This paper has been published by Cambridge University Press in "Geological magazine"(146: 3 (2009) 353-367) http://journals.cambridge.org/action/displayJournal?jid=GEO Additional information: Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in DRO • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full DRO policy for further details. Durham University Library, Stockton Road, Durham DH1 3LY, United Kingdom Tel : +44 (0)191 334 3042 | Fax : +44 (0)191 334 2971 https://dro.dur.ac.uk Geol. Mag. 146 (3), 2009, pp. 353–367. c 2009 Cambridge University Press 353 doi:10.1017/S0016756809005974 Printed in the United Kingdom Understanding the offshore flood basalt sequence using onshore volcanic facies analogues: an example from the Faroe–Shetland basin ∗ ∗ ∗ DOUGAL A. -

Subglacial and Submarine Volcanism in Iceland
Mars Polar Science 2000 4078.pdf SUBGLACIAL AND SUBMARINE VOLCANISM IN ICELAND. S. P. Jakobsson, Icelandic Inst. of Natural His- tory, P. O. Box 5320, 125 Reykjavik, Iceland Introduction: Iceland is the largest landmass ex- mounds, ridges and tuyas [5]. The thickness of basal posed along the Mid-Ocean Ridge System. It has been basaltic pillow lava piles often exceeds 60-80 meters constructed over the past 16 Ma by basaltic to silicic and a 300 m thick section has been reported. Pillow volcanic activity occurring at the Mid-Atlantic Ridge, lavas may also form lenses or pods at a higher level in and is topographically elevated because of the abundant the volcanoes. igneous material produced in association with the Ice- It has been suggested that at a water depth less than land hot spot, the center of which is thought to be lo- approximately 100-150 m, basaltic phreatic explosions cated beneath Vatnajokull glacier [1]. The axial rift produce hydroclastites. It appears feasible to subdivide zones which run through Iceland from southwest to the hyaloclastites of the Icelandic ridges and tuyas, ge- northeast are in direct continuation of the crestal zones netically into two main types. A substantial part of the of the Mid Atlantic Ridge and are among the most ac- base of the submarine Surtsey tuya is poorly bedded, tive volcanic zones on Earth. unsorted, hydroclastite, which probably was quenched Subglacial Volcanism: Volcanic accumulations of and rapidly accumulated below the seawater level with- hyaloclastites which are deposits formed by the intru- out penetrating the surface [6]. Only 1-2 % of the vol- sion of lava beneath water or ice and the consequent ume of extruded material in the 1996 Gjalp eruption fell shattering into small angular vitric particles, combined as air-fall tephra, the bulk piled up below the ice [4]. -

Ocean Drilling Program Scientific Results Volume
Taylor, B., Fujioka, K., et al., 1992 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 126 30. CHARACTERIZATION AND CORRELATION OF MEGASCOPIC TEPHRAS IN SITE 792 CORES FROM THE IZU-OGASAWARA FOREARC BASIN (JAPAN) BY TRACE ELEMENTS AND 87Sr/86Sr AND 143Nd/144Nd ISOTOPES1 Per {Cristian Egeberg,2 Arild O. Brunfelt,3 and Ame S. Stabel3 ABSTRACT Glass shards from 26 megascopic tephras (1-29 cm thick) were separated and analyzed for 26 elements and 87Sr/86Sr and 143Nd/144Nd isotope ratios. The isotope data allowed us to assess the degree of alteration of the samples. The separates contain less than 10% of palagonitized glass or secondary alteration minerals. Correlation diagrams and multivariate analyses have been used to characterize the glass shards in a tectonic setting and group the tephras according to their magmatic affinity. Four tholeiitic groups, two calc-alkaline groups, and one group with glass of shoshonitic affinity can be distinguished. The tholeiitic groups form a time series; however, judging from the rare-earth-element patterns and the temporal variations of the Ce/Yb ratios, the evolution is not unidirectional from strong to weak tholeiitic affinity. The tephras have been correlated with volcanism along the Izu-Ogasawara and Ryukyu arcs by means of trace element distribution, 87Sr/86Sr ratios, and their magmatic affinity. The tholeiitic layers were derived from volcanoes on the Izu-Ogasawara Arc, most probably from the volcanic island Hachijojima. Only one group of the calc-alkaline layers can have derived from the Izu-Ogasawara Arc. The other calc-alkaline layers and the two layers with shoshonitic affinity probably derive from volcanism along the Ryukyu Arc. -

The Purcell Lava Glacier National Park, Montana
UNITED STATES DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY The Purcell Lava Glacier National Park, Montana by Robert G. McGimsey 1 Open-File Report 85-0543 1985 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editoral standards and stratigraphic nomenclature Denver, Colorado CONTENTS Page LIST OF FIGURES.......................................................... iii LIST OF TABLES........................................................... vii ABSTRACT................................................................. 1 ACKNOWLEDGMENTS.......................................................... 2 CHAPTER I. INTRODUCTION..................................................... 3 Purpose of Investigation....................................... 3 Location and Geologic Setting.................................. 3 Previous Work.................................................. 4 Field and Laboratory Methods................................... 5 II. DIVISION AND DESCRIPTION OF FACIES............................... 6 Introduction................................................... 6 Pillow Lava Facies............................................. 6 Two-Dimensional Pillow Exposures............................. 7 Three-Dimensional Pillow Exposures........................... 9 Isolated- and Broken-Pillow Breccia.......................... 9 Coalesced Pillows and the Upper Contact...................... 10 Hyaloclastite Breccia........................................ 10 Intercalated Sediment....................................... -
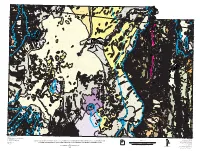
Geologic Map of Delta 30' X 60' Quadrangle
113 00' 33 R 10 W 3 -Cpm 4 R 9 W p-Cm - R 8 W 45' 35 Qla P Cp Qla Qvb 3 B Qla QTlf Qlm 5 6 R 7 W Qla Qaf1 3 R 5 W 3 Qpm Qed R 6 W 7 30' 8 3 A Qaf1 -Cp Qla Qaf1 Qes Qlm Qlf Qpm R 4 W 9 15' Qlg Qed Qed Qlf Qla Qaf1 Qed Qed Qla Qpm Qed Qed P Qlm QTlf Qlm Qed p-Cc Qaf2 Qaf Qlf Qed Qdf Tdr Qaf1 1 Qed Qed -Cpm p-Cm Qla Qla Qla Qlf Qed Qed p-Cm Tdi Qaf1 Qla Qed Qed p-Cc B Qdf Trk Qaf2 Qlm Qaf1 Qed 85 86 -Cdh -Cp Qed Qaf1 Qaf1 30 Qaf Qlf Qed Qed -Cp Tdr 1 Qed B Qla Qpm Qdf B -Cpm -Cpm P Qlf Qed Qal -Cdh -Cp Qlm Qla 1 p-Cm -Cp Qaf1 Qed Qlf Qal - Qed 2 -Cpm Cdh ? Qdf Qdf Qdg p-Cc -Cdh Qla Qla Qlf/Qal /QTlf Qdg/Qdf Qal2 60 -Cum -Cp 25 3 Qed p-Ci 45 -Cww - Qla Qed Qed Qed Qdf -Cp Cpm -Cpm Qed Qdg Kc Qla Qpm Qed -Cpm 87 -Cdh -Cdh Qaf1 Qed Qdg -Cdh -Cp Qpm -Cww Qla Qdg Qes Qdf B -Cp Qed Qed Qed Qdf Qaf1 - P Cp B Qdg 20 -Cww Qla Qpm Qes p-Cm -Cp -Cww 30 Qaf -Cum Qaf 1 2 -Cp Qla Qed Qal2 -Cdh -Cdh -Cdh Qed Qes p-Cm Qed Qed Qaf1 -Cpm -Cmp Qla P Qed Qal1 -Cww Qms Qla 78-1 -Cp Kc -Cdh Qed Qed Qdf - p-Cm -Cww 86 39 30' Qaf2 -Cmp Cmp -Cww -Cum 80 B Qaf1 Qlm Qed Tdr Qed 64 Tdr Qaf Qlg Qdf Qdf Qdf 60 2 Qal2 Qaf1 Qlg Qed p-Cm Qaf2 Qla P Qal Tdr Qlg Qaf Qla 1 R 3 W 40 Qla 1 Qes Qed 1 825 R 2 W 41 1 850 000 FEET 112 00' T 15 S -Cww QTaf QTas Qdf 39 30' Qlm Qed Qaf -Cdh QTaf Qdf 1 -Cum Qaf1 Qdf 15 Tf Qaf2 Qaf Qaf1 ? QTaf Qac 2 Qaf -Cp QTaf Qlg Tdr 2 Qal p-Cc Qms Qaf1 B Tbsk 1 85 QTaf QTaf Qdf Tf B Qlg Qdf TKn Qaf Qdf Qal Qes Qal2 Qla p-Cc Qaf2 Qms 1 Tf Qaf Tdr Qla Qla 2 Qes Qed QTas 2 Qaf1 -Cdh QTaf Qaf Qed p-Cm - Tf Qlg Qaf2 1 Tbsk -Cp Cww QTaf - 40 TKn QTaf QTaf -

Subglacial to Emergent Volcanism at Shield Nunatak, Mt. Melbourne Volcanic Field, Antarctica
Polarforschung 57 1/2: 27-41, 1987 Subglacial to Emergent Volcanism at Shield Nunatak, Mt. Melbourne Volcanic Field, Antarctica By G. Wörner and L. Viereck* Summary: Sections through Shield Nunatak vo1cano, an alkali basaltic subglacial table-mountain from the Mt. Melbourne Volcanic Field, are descrlbed. Resting on a base of older lava flows and a fossile tillite iayer, the volcano is formed mainly by massive ash and lapilli (mass flow) deposits while pillow lavas are absent. The recurrent stage of emergence from subglacial to subaerial conditions is characterized by de velopment of tuff rings from explosive interaction between magma and melt water, complex ash- and lapilli deposits, including lava flows brecciated by water-interaction and a subaerial basaltie ash-flow deposit. The sequence is capped by subaerial tuff rings, lava flows, scoria cones and reworked tephra. Available data on subaquatlc explosive voJcanism indicate that early Shield Nunatak basaltic lavas erupted un der less than 300 m of ice cover: later Iavas erupted under shallow-water and subaerial conditions. Shield Nunatak probably formed during a glacial period (possibly the last) when the ice thtckness in the Mt. Melbourne area must have been at least 200 m greater than at present. The volcano has suffered only minor glacial erosion and still almost has its original shape. Zusammenfassung: Es werden Profile beschrieben durch einen alkalibasaltischen subglazialen Vulkan (Shield Nunatak) im Mt. Melbourne Vulkanfeld. Die Unterlage des Vulkans wird von einer Serie subaerischer Lavaströme intermediärer Zusammensetzung (Mugearit) gebildet. Über einer Lage von Paleo-Till bilden massive Aschenablagerungen die Basis der subglazialen Sequenz. Kissenlaven fehlen. -

Classification of Volcanic Ash Particles Using a Convolutional Neural
Classification of volcanic ash particles using a convolutional neural network and probability Daigo Shoji1∗, Rina Noguchi2, Shizuka Otsuki3, Hideitsu Hino4 1. Earth-Life Science Institute, Tokyo Institute of Technology, 2-12-1 Ookayama, Meguro-ku, Tokyo 2. Volcanic Fluid Research Center, School of Science, Tokyo Institute of Technology, 2-12-1, Ookayama, Meguro-ku, Tokyo 3.Geological Survey of Japan, AIST, 1-1-1 Higashi, Tsukuba, Ibaraki 4.The Institute of Statistical Mathematics,10-3 Midori-cho, Tachikawa, Tokyo ∗ Corresponding author: [email protected] arXiv:1805.12353v1 [physics.geo-ph] 31 May 2018 1 Abstract Analyses of volcanic ash are typically performed either by qualitatively classifying ash particles by eye or by quantitatively parameterizing its shape and texture. While complex shapes can be classified through qualitative analyses, the results are subjective due to the difficulty of categorizing complex shapes into a single class. Although quantitative analyses are objective, selection of shape parame- ters is required. Here, we applied a convolutional neural network (CNN) for the classification of volcanic ash. First, we defined four basal particle shapes (blocky, vesicular, elongated, rounded) generated by different eruption mechanisms (e.g., brittle fragmentation), and then trained the CNN using particles composed of only one basal shape. The CNN could recognize the basal shapes with over 90% accuracy. Using the trained network, we classified ash particles composed of mul- tiple basal shapes based on the output of the network, which can be interpreted as a mixing ratio of the four basal shapes. Clustering of samples by the averaged probabilities and the intensity is consistent with the eruption type. -

Basaltic Glasses from Iceland and the Deep Sea: Natural Analogues to Borosilicate Nuclear Waste-Form Glass
Basaltic glasses from Iceland and the deep sea: Natural analogues to borosilicate nuclear waste-form glass. MicliMlJ.J«rcinovfc and Rodney C.Ewing D«c«mb«r,1987 BASALTIC OLACSBI FROM ICBLAHD AVD THE DB» SBA: ITOBAX. AMALOGUBf TO BOROflLICATB MUCL1AB WMT1-F0RM GLASS Michael J. J«rcinovic and Rodn«y C. Ewing D«c«mb«r, 1987 D«parta«nt of Geology Th« University of New Mexico Albuquerque, New Mexico USA 87131 11 list of Tables iv list of Figures vi Suenery xiii Abstract xvi 1 introduction 1 1.1 Alteration 6 1.1.1 Palagonitizaticn 6 1.1.2 Palagcnitizaticn Rates 9 1.1.3 Secondary Mineralization 13 1.2 Samples 21 1.2.1 Iceland 21 1.2.2 Dredge Sanples 26 1.2.3 Drill Core Samples 26 2 Techniques 29 2.1 Thin Section Preparation 29 2.2 Scanning Electron Microscopy 31 2.3 X-Ray Diffraction 31 2.4 Electron Microprobe Analysis 32 2.5 Analytical Electron Microscopy 34 3 Results 35 3.1 Icelani 35 3.1.1 General cements 35 3.1.2 Fresh Mater Alteration 36 3.1.2.1 Pleistocene Snhjiftrtnl Volcanic» 37 3.1.2.1.1 Palagonite 37 3.1.2.1.2 Cssentation 42 3.1.2.2 Tungufell 55 3.1.2.2.1 Palagcnite 55 3.1.2.2.2 Oawntation 68 3.1.3 Seawater Alteration 72 3.1.3.1 General Conomts 72 3.1.3.1.1 Palagcnite 76 3.1.3.1.2 Cementation , 92 11.' 3.2 EKedge Sau&m 107 3.2.1 ROagonite 107 3.2.2 OsasntiiHin 117 3.3 Erill Om Saaples 128 3.3.1 ffelagcnite 128 3.3.2 t 3.4 Analytical Electron Microscopy 144 3.4.1 Saaple Description 144 3.4.2 Analytical Ilectrcn Micxceoopy 147 3.4.2.1 OSMI 113521-69 147 3.4.2.2 UGM1 113715 153 3.4.3 conclusion* 156 4 Discussion 158 4.1 ROagonite 158 4.2 Secondary Mineral Authigenasis, Solution Concentrations, and Mass Balance 180 4.3 Alteration Rates 200 5 Conclusions 206 5.1 Corrosion Machanisn 206 5.2 Alteration Products 207 5.3 Mass Balance 209 5.4 Alteration Rates 210 Acknowledgements 212 213 iv Table 1.