Structural Basis for Binding the TREX2 Complex to Nuclear Pores, GAL1 Localisation and Mrna Export Divyang Jani, Eugene Valkov and Murray Stewart*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trex2 Enables Spontaneous Sister Chromatid Exchanges Without Facilitating DNA Double-Strand Break Repair
INVESTIGATION Trex2 Enables Spontaneous Sister Chromatid Exchanges Without Facilitating DNA Double-Strand Break Repair Lavinia C. Dumitrache,*,1,2 Lingchuan Hu,*,1 Mi Young Son,* Han Li,*,3 Austin Wesevich,* Ralph Scully,† Jeremy Stark,‡ and Paul Hasty*,4 *Department of Molecular Medicine/Institute of Biotechnology, University of Texas Health Science Center, San Antonio, Texas 78245-3207, yDepartment of Medicine, Harvard Medical School, Beth Israel Deaconess Medical Center Division of Hematology– Oncology/Cancer Biology Program, Boston, Massachusetts 02115, and ‡Department of Cancer Biology, Division of Radiation Biology, Beckman Research Institute of the City of Hope, Duarte, California 91010 ABSTRACT Trex2 is a 39 / 59 exonuclease that removes 39-mismatched sequences in a biochemical assay; however, its biological function remains unclear. To address biology we previously generated trex2null mouse embryonic stem (ES) cells and expressed in these cells wild-type human TREX2 cDNA (Trex2hTX2) or cDNA with a single-amino-acid change in the catalytic domain (Trex2H188A) or in the DNA-binding domain (Trex2R167A). We found the trex2null and Trex2H188A cells exhibited spontaneous broken chromosomes and trex2null cells exhibited spontaneous chromosomal rearrangements. We also found ectopically expressed human TREX2 was active at the 39 ends of I-SceI–induced chromosomal double-strand breaks (DSBs). Therefore, we hypothesized Trex2 participates in DNA DSB repair by modifying 39 ends. This may be especially important for ends with damaged nucleotides. Here we present data that are unexpected and prompt a new model. We found Trex2-altered cells (null, H188A, and R167A) were not hypersensitive to campto- thecin, a type-1 topoisomerase inhibitor that induces DSBs at replication forks. -

Redesign of Extensive Protein–DNA Interfaces of Meganucleases Using Iterative Cycles of in Vitro Compartmentalization
Redesign of extensive protein–DNA interfaces of meganucleases using iterative cycles of in vitro compartmentalization Ryo Takeuchi, Michael Choi, and Barry L. Stoddard1 Division of Basic Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA 98109 Edited by David Baker, University of Washington, Seattle, WA, and approved February 13, 2014 (received for review November 8, 2013) LAGLIDADG homing endonucleases (meganucleases) are sequence- residues. Although an industrial-scale engineering pipeline for specific DNA cleavage enzymes used for genome engineering. altering meganuclease specificity has been developed (6), the Recently, meganucleases fused to transcription activator-like effec- specialized knowledge and technology required for that ap- tors have been demonstrated to efficiently introduce targeted proach generally precludes their use by academic laboratories. genome modifications. However, retargeting meganucleases to In addition, a recent study has demonstrated that MegaTALs genomic sequences of interest remains challenging because it usu- (fusions of TAL effector DNA binding regions to meganucleases) ally requires extensive alteration of a large number of amino acid can induce a high level of targeted gene modification in human residues that are situated in and near the DNA interface. Here we cells (21). However, the utility of this platform still depends upon describe an effective strategy to extensively redesign such an the development of efficient strategies to engineer meganucleases extensive biomolecular interface. -

5' Exonuclease TREX1 in Human Disease
Edinburgh Research Explorer New roles for the major human 3'-5' exonuclease TREX1 in human disease Citation for published version: Kavanagh, D, Spitzer, D, Kothari, PH, Shaikh, A, Liszewski, MK, Richards, A & Atkinson, JP 2008, 'New roles for the major human 3'-5' exonuclease TREX1 in human disease', Cell Cycle, vol. 7, no. 12, pp. 1718- 25. Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: Cell Cycle General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 26. Sep. 2021 NIH Public Access Author Manuscript Cell Cycle. Author manuscript; available in PMC 2010 February 19. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Cell Cycle. 2008 June 15; 7(12): 1718±1725. New roles for the major human 3'–5' exonuclease TREX1 in human disease David Kavanagh1, Dirk Spitzer2, Parul H. Kothari2, Aisha Shaikh2, M. Kathryn -

Multifaceted Role of TREX2 in the Skin Defense Against UV-Induced Skin Carcinogenesis
www.impactjournals.com/oncotarget/ Oncotarget, Vol. 6, No. 26 Multifaceted role of TREX2 in the skin defense against UV-induced skin carcinogenesis Joan Manils1, Diana Gómez1, Mercè Salla-Martret1, Heinz Fischer2, Jason M. Fye3, Elena Marzo1, Laura Marruecos1, Inma Serrano1, Rocío Salgado4, Juan P. Rodrigo5, Juana M. Garcia-Pedrero5, Anna M. Serafin6, Xavier Cañas6, Carmen Benito7, Agustí Toll4, Sònia-Vanina Forcales8, Fred W. Perrino3, Leopold Eckhart2, Concepció Soler1 1 Departament de Patologia i Terapèutica Experimental, Facultat de Medicina, Campus de Bellvitge, Universitat de Barcelona, L’Hospitalet de Llobregat, Barcelona, Spain 2 Research Division of Biology and Pathobiology of the Skin, Department of Dermatology, Medical University of Vienna, Vienna, Austria 3Department of Biochemistry, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA 4Dermatology Departament, Hospital del Mar, Barcelona, Spain 5 Department of Otolaryngology, Hospital Universitario Central de Asturias, Instituto Universitario de Oncología del Principado de Asturias (IUOPA), Universidad de Oviedo, Oviedo, Spain 6Plataforma de Recerca Aplicada en Animal de Laboratori, Parc Científic de Barcelona, Barcelona, Spain 7Protecció Radiològica, Universitat de Barcelona, Barcelona, Spain 8Institute of Predictive and Personalized Medicine of Cancer, Badalona, Barcelona, Spain Correspondence to: Concepció Soler, e-mail: [email protected] Keywords: TREX2, skin carcinogenesis, UV radiation, DNA damage, inflammation Received: April 23, 2015 Accepted: June 02, 2015 Published: June 15, 2015 ABSTRACT TREX2 is a 3′-DNA exonuclease specifically expressed in keratinocytes. Here, we investigated the relevance and mechanisms of TREX2 in ultraviolet (UV)-induced skin carcinogenesis. TREX2 expression was up-regulated by chronic UV exposure whereas it was de-regulated or lost in human squamous cell carcinomas (SCCs). -

RNA Sequencing Analyses Reveal Differentially Expressed Genes and Pathways As Notch2 Targets in B-Cell Lymphoma
www.oncotarget.com Oncotarget, 2020, Vol. 11, (No. 48), pp: 4527-4540 Research Paper RNA sequencing analyses reveal differentially expressed genes and pathways as Notch2 targets in B-cell lymphoma Ashok Arasu1, Pavithra Balakrishnan1 and Thirunavukkarasu Velusamy1 1Department of Biotechnology, School of Biotechnology and Genetic Engineering, Bharathiar University, Coimbatore, India Correspondence to: Thirunavukkarasu Velusamy, email: [email protected] Keywords: SMZL; Notch2; RNA sequencing; PI3K/AKT; NF-kB Received: August 04, 2020 Accepted: October 17, 2020 Published: December 01, 2020 Copyright: © 2020 Arasu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Splenic marginal zone lymphoma (SMZL) is a low grade, indolent B-cell neoplasm that comprises approximately 10% of all lymphoma. Notch2, a pivotal gene for marginal zone differentiation is found to be mutated in SMZL. Deregulated Notch2 signaling has been involved in tumorigenesis and also in B-cell malignancies. However the role of Notch2 and the downstream pathways that it influences for development of B-cell lymphoma remains unclear. In recent years, RNA sequencing (RNA-Seq) has become a functional and convincing technology for profiling gene expression and to discover new genes and transcripts that are involved in disease development in a single experiment. In the present study, using transcriptome sequencing approach, we have identified key genes and pathways that are probably the underlying cause in the development of B-cell lymphoma. We have identified a total of 15,083 differentially expressed genes (DEGs) and 1067 differentially expressed transcripts (DETs) between control and Notch2 knockdown B cells. -

Differential Mechanisms of Tolerance to Extreme Environmental
www.nature.com/scientificreports OPEN Diferential mechanisms of tolerance to extreme environmental conditions in tardigrades Dido Carrero*, José G. Pérez-Silva , Víctor Quesada & Carlos López-Otín * Tardigrades, also known as water bears, are small aquatic animals that inhabit marine, fresh water or limno-terrestrial environments. While all tardigrades require surrounding water to grow and reproduce, species living in limno-terrestrial environments (e.g. Ramazzottius varieornatus) are able to undergo almost complete dehydration by entering an arrested state known as anhydrobiosis, which allows them to tolerate ionic radiation, extreme temperatures and intense pressure. Previous studies based on comparison of the genomes of R. varieornatus and Hypsibius dujardini - a less tolerant tardigrade - have pointed to potential mechanisms that may partially contribute to their remarkable ability to resist extreme physical conditions. In this work, we have further annotated the genomes of both tardigrades using a guided approach in search for novel mechanisms underlying the extremotolerance of R. varieornatus. We have found specifc amplifcations of several genes, including MRE11 and XPC, and numerous missense variants exclusive of R. varieornatus in CHEK1, POLK, UNG and TERT, all of them involved in important pathways for DNA repair and telomere maintenance. Taken collectively, these results point to genomic features that may contribute to the enhanced ability to resist extreme environmental conditions shown by R. varieornatus. Tardigrades are small -

Analyzing the Mirna-Gene Networks to Mine the Important Mirnas Under Skin of Human and Mouse
Hindawi Publishing Corporation BioMed Research International Volume 2016, Article ID 5469371, 9 pages http://dx.doi.org/10.1155/2016/5469371 Research Article Analyzing the miRNA-Gene Networks to Mine the Important miRNAs under Skin of Human and Mouse Jianghong Wu,1,2,3,4,5 Husile Gong,1,2 Yongsheng Bai,5,6 and Wenguang Zhang1 1 College of Animal Science, Inner Mongolia Agricultural University, Hohhot 010018, China 2Inner Mongolia Academy of Agricultural & Animal Husbandry Sciences, Hohhot 010031, China 3Inner Mongolia Prataculture Research Center, Chinese Academy of Science, Hohhot 010031, China 4State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China 5Department of Biology, Indiana State University, Terre Haute, IN 47809, USA 6The Center for Genomic Advocacy, Indiana State University, Terre Haute, IN 47809, USA Correspondence should be addressed to Yongsheng Bai; [email protected] and Wenguang Zhang; [email protected] Received 11 April 2016; Revised 15 July 2016; Accepted 27 July 2016 Academic Editor: Nicola Cirillo Copyright © 2016 Jianghong Wu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Genetic networks provide new mechanistic insights into the diversity of species morphology. In this study, we have integrated the MGI, GEO, and miRNA database to analyze the genetic regulatory networks under morphology difference of integument of humans and mice. We found that the gene expression network in the skin is highly divergent between human and mouse. -

Table S1. 103 Ferroptosis-Related Genes Retrieved from the Genecards
Table S1. 103 ferroptosis-related genes retrieved from the GeneCards. Gene Symbol Description Category GPX4 Glutathione Peroxidase 4 Protein Coding AIFM2 Apoptosis Inducing Factor Mitochondria Associated 2 Protein Coding TP53 Tumor Protein P53 Protein Coding ACSL4 Acyl-CoA Synthetase Long Chain Family Member 4 Protein Coding SLC7A11 Solute Carrier Family 7 Member 11 Protein Coding VDAC2 Voltage Dependent Anion Channel 2 Protein Coding VDAC3 Voltage Dependent Anion Channel 3 Protein Coding ATG5 Autophagy Related 5 Protein Coding ATG7 Autophagy Related 7 Protein Coding NCOA4 Nuclear Receptor Coactivator 4 Protein Coding HMOX1 Heme Oxygenase 1 Protein Coding SLC3A2 Solute Carrier Family 3 Member 2 Protein Coding ALOX15 Arachidonate 15-Lipoxygenase Protein Coding BECN1 Beclin 1 Protein Coding PRKAA1 Protein Kinase AMP-Activated Catalytic Subunit Alpha 1 Protein Coding SAT1 Spermidine/Spermine N1-Acetyltransferase 1 Protein Coding NF2 Neurofibromin 2 Protein Coding YAP1 Yes1 Associated Transcriptional Regulator Protein Coding FTH1 Ferritin Heavy Chain 1 Protein Coding TF Transferrin Protein Coding TFRC Transferrin Receptor Protein Coding FTL Ferritin Light Chain Protein Coding CYBB Cytochrome B-245 Beta Chain Protein Coding GSS Glutathione Synthetase Protein Coding CP Ceruloplasmin Protein Coding PRNP Prion Protein Protein Coding SLC11A2 Solute Carrier Family 11 Member 2 Protein Coding SLC40A1 Solute Carrier Family 40 Member 1 Protein Coding STEAP3 STEAP3 Metalloreductase Protein Coding ACSL1 Acyl-CoA Synthetase Long Chain Family Member 1 Protein -
Downloaded from the Mouse Lysosome Gene Database, Mlgdb
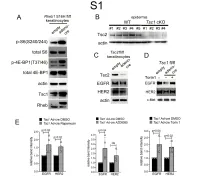
1 Supplemental Figure Legends 2 3 Supplemental Figure S1: Epidermal-specific mTORC1 gain-of-function models show 4 increased mTORC1 activation and down-regulate EGFR and HER2 protein expression in a 5 mTORC1-sensitive manner. (A) Immunoblotting of Rheb1 S16H flox/flox keratinocyte cultures 6 infected with empty or adenoviral cre recombinase for markers of mTORC1 (p-S6, p-4E-BP1) 7 activity. (B) Tsc1 cKO epidermal lysates also show decreased expression of TSC2 by 8 immunoblotting of the same experiment as in Figure 2A. (C) Immunoblotting of Tsc2 flox/flox 9 keratinocyte cultures infected with empty or adenoviral cre recombinase showing decreased EGFR 10 and HER2 protein expression. (D) Expression of EGFR and HER2 was decreased in Tsc1 cre 11 keratinocytes compared to empty controls, and up-regulated in response to Torin1 (1µM, 24 hrs), 12 by immunoblot analyses. Immunoblots are contemporaneous and parallel from the same biological 13 replicate and represent the same experiment as depicted in Figure 7B. (E) Densitometry 14 quantification of representative immunoblot experiments shown in Figures 2E and S1D (r≥3; error 15 bars represent STDEV; p-values by Student’s T-test). 16 17 18 19 20 21 22 23 Supplemental Figure S2: EGFR and HER2 transcription are unchanged with epidermal/ 24 keratinocyte Tsc1 or Rptor loss. Egfr and Her2 mRNA levels in (A) Tsc1 cKO epidermal lysates, 25 (B) Tsc1 cKO keratinocyte lysates and(C) Tsc1 cre keratinocyte lysates are minimally altered 26 compared to their respective controls. (r≥3; error bars represent STDEV; p-values by Student’s T- 27 test). -

S41467-019-13965-X.Pdf
ARTICLE https://doi.org/10.1038/s41467-019-13965-x OPEN Genome-wide CRISPR screen identifies host dependency factors for influenza A virus infection Bo Li1,2, Sara M. Clohisey 3, Bing Shao Chia1,2, Bo Wang 3, Ang Cui2,4, Thomas Eisenhaure2, Lawrence D. Schweitzer2, Paul Hoover2, Nicholas J. Parkinson3, Aharon Nachshon 5, Nikki Smith3, Tim Regan 3, David Farr3, Michael U. Gutmann6, Syed Irfan Bukhari7, Andrew Law 3, Maya Sangesland8, Irit Gat-Viks2,5, Paul Digard 3, Shobha Vasudevan7, Daniel Lingwood8, David H. Dockrell9, John G. Doench 2, J. Kenneth Baillie 3,10* & Nir Hacohen 2,11* 1234567890():,; Host dependency factors that are required for influenza A virus infection may serve as therapeutic targets as the virus is less likely to bypass them under drug-mediated selection pressure. Previous attempts to identify host factors have produced largely divergent results, with few overlapping hits across different studies. Here, we perform a genome-wide CRISPR/ Cas9 screen and devise a new approach, meta-analysis by information content (MAIC) to systematically combine our results with prior evidence for influenza host factors. MAIC out- performs other meta-analysis methods when using our CRISPR screen as validation data. We validate the host factors, WDR7, CCDC115 and TMEM199, demonstrating that these genes are essential for viral entry and regulation of V-type ATPase assembly. We also find that CMTR1, a human mRNA cap methyltransferase, is required for efficient viral cap snatching and regulation of a cell autonomous immune response, and provides synergistic protection with the influenza endonuclease inhibitor Xofluza. 1 Harvard University Virology Program, Harvfvard Medical School, Boston MA02142, USA. -
Sirna Screening Identifies Differences in the Fanconi Anemia
Oncogene (2013) 32, 5458–5470 OPEN & 2013 Macmillan Publishers Limited All rights reserved 0950-9232/13 www.nature.com/onc ORIGINAL ARTICLE siRNA screening identifies differences in the Fanconi anemia pathway in BALB/c-Trp53 þ / À with susceptibility versus C57BL/6-Trp53 þ / À mice with resistance to mammary tumors MBo¨ hringer1,4, K Obermeier1,4, N Griner2, D Waldraff1, E Dickinson2, K Eirich3, D Schindler3, M Hagen2, DJ Jerry2,5 and L Wiesmu¨ ller1,5 BALB/c mice heterozygous for Trp53 develop a high proportion of spontaneous mammary tumors, a phenotype distinct from other mouse strains. BALB/c-Trp53 þ / À female mice, thus, resemble the hereditary Li-Fraumeni syndrome (LFS) characterized by early- onset of breast cancer, even though LFS involves TP53 mutations, which may involve not only loss- but also gain-of-function. Previous analysis of tumors in BALB/c-Trp53 þ / À females showed frequent loss of heterozygosity involving the wild-type allele of Trp53 and displayed characteristics indicative of mitotic recombination. Critical involvement of DNA double-strand break (DSB) repair dysfunction, particularly of homologous recombination (HR), was also noticed in the etiology of human breast cancer. To better define functional alterations in BALB/c-Trp53 þ / À mice, we applied a fluorescence-based DSB repair assay on mouse embryonic fibroblasts (MEFs) from BALB/c-Trp53 þ / À versus C57BL/6J-Trp53 þ / À mice. This approach revealed deregulation of HR but not non-homologous end-joining (NHEJ) in BALB/c-Trp53 þ / À , which was further confirmed for mammary epithelial cells. Screening of a small interfering RNA-library targeting DSB repair, recombination, replication and signaling genes, identified 25 genes causing differences between homologous DSB repair in the two strains upon silencing. -

The Seventh International Conference on Bioinformatics of Genome Regulation and Structure\Systems Biology
RUSSIAN ACADEMY OF SCIENCES SIBERIAN BRANCH INSTITUTE OF CYTOLOGY AND GENETICS THE SEVENTH INTERNATIONAL CONFERENCE ON BIOINFORMATICS OF GENOME REGULATION AND STRUCTURE\SYSTEMS BIOLOGY Abstracts BGRS\SB’10 Novosibirsk, Russia June 20–27, 2010 Novosibirsk 2010 INTERNATIONAL PROGRAM COMMITTEE Nikolay Kolchanov, Institute of Cytology and Genetics SB RAS, Novosibirsk, Russia (Chairman of the Conference) Ralf Hofestadt, University of Bielefeld, Germany (Co-Chairman of the Conference) Konstantin Skryabin, “Bioingineering” Center, RAS, Moscow, Russia (Co-Chairman of the Conference) Dagmara Furman, Institute of Cytology and Genetics SB RAS, Novosibirsk, (Conference Scientifi c Secretary) Dmitry Afonnikov, Institute of Cytology and Genetics SB RAS, Novosibirsk, Russia Lev Beloussov, Moscow State University, Moscow, Russia Sergey Deyev, Shemyakin/Ovchinnikov Institute of Bioorganic Chemistry RAS, Moscow, Russia Roman Efremov, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry RAS, Moscow, Russia А. Fazel Famili, University of Ottawa, IIT/ITI - National Research Council Canada, Ottawa, Canada Igor Goryanin, Edinburgh Centre for Bioinformatics, University of Edinburgh, Edinburgh, United Kingdom Charlie Hodgman, Multidisciplinary Centre for Integrative Biology, Centre of Plant Integrative Biology, University of Nottingham, UK Vladimir Ivanisenko, Institute of Cytology and Genetics, SB RAS, Novosibirsk, Russia Alexis Ivanov, Institute of Biomedical Chemistry RAMS, Moscow, Russia Yoichiro Iwakura, Center for Experimental Medicine and Systems Biology,