Deep Sequencing of Subcellular RNA Fractions Shows Splicing to Be Predominantly Co-Transcriptional in the Human Genome but Inefficient for Lncrnas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coupling of Spliceosome Complexity to Intron Diversity
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.19.436190; this version posted March 20, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Coupling of spliceosome complexity to intron diversity Jade Sales-Lee1, Daniela S. Perry1, Bradley A. Bowser2, Jolene K. Diedrich3, Beiduo Rao1, Irene Beusch1, John R. Yates III3, Scott W. Roy4,6, and Hiten D. Madhani1,6,7 1Dept. of Biochemistry and Biophysics University of California – San Francisco San Francisco, CA 94158 2Dept. of Molecular and Cellular Biology University of California - Merced Merced, CA 95343 3Department of Molecular Medicine The Scripps Research Institute, La Jolla, CA 92037 4Dept. of Biology San Francisco State University San Francisco, CA 94132 5Chan-Zuckerberg Biohub San Francisco, CA 94158 6Corresponding authors: [email protected], [email protected] 7Lead Contact 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.19.436190; this version posted March 20, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. SUMMARY We determined that over 40 spliceosomal proteins are conserved between many fungal species and humans but were lost during the evolution of S. cerevisiae, an intron-poor yeast with unusually rigid splicing signals. We analyzed null mutations in a subset of these factors, most of which had not been investigated previously, in the intron-rich yeast Cryptococcus neoformans. -

Nuclear Expression of a Group II Intron Is Consistent with Spliceosomal Intron Ancestry
Downloaded from genesdev.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press Nuclear expression of a group II intron is consistent with spliceosomal intron ancestry Venkata R. Chalamcharla, M. Joan Curcio, and Marlene Belfort1 Center for Medical Sciences, Wadsworth Center, New York State Department of Health, Albany, New York 12208, USA; and School of Public Health, State University of New York at Albany, Albany, New York 12201, USA Group II introns are self-splicing RNAs found in eubacteria, archaea, and eukaryotic organelles. They are mechanistically similar to the metazoan nuclear spliceosomal introns; therefore, group II introns have been invoked as the progenitors of the eukaryotic pre-mRNA introns. However, the ability of group II introns to function outside of the bacteria-derived organelles is debatable, since they are not found in the nuclear genomes of eukaryotes. Here, we show that the Lactococcus lactis Ll.LtrB group II intron splices accurately and efficiently from different pre-mRNAs in a eukaryote, Saccharomyces cerevisiae. However, a pre-mRNA harboring a group II intron is spliced predominantly in the cytoplasm and is subject to nonsense-mediated mRNA decay (NMD), and the mature mRNA from which the group II intron is spliced is poorly translated. In contrast, a pre-mRNA bearing the Tetrahymena group I intron or the yeast spliceosomal ACT1 intron at the same location is not subject to NMD, and the mature mRNA is translated efficiently. Thus, a group II intron can splice from a nuclear transcript, but RNA instability and translation defects would have favored intron loss or evolution into protein-dependent spliceosomal introns, consistent with the bacterial group II intron ancestry hypothesis. -
Primepcr™Assay Validation Report
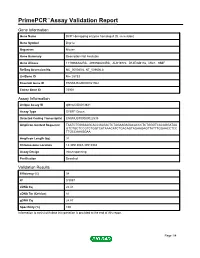
PrimePCR™Assay Validation Report Gene Information Gene Name DCP1 decapping enzyme homolog A (S. cerevisiae) Gene Symbol Dcp1a Organism Mouse Gene Summary Description Not Available Gene Aliases 1110066A22Rik, 4930568L04Rik, AU019772, D14Ertd817e, Mitc1, SMIF RefSeq Accession No. NC_000080.6, NT_039606.8 UniGene ID Mm.28733 Ensembl Gene ID ENSMUSG00000021962 Entrez Gene ID 75901 Assay Information Unique Assay ID qMmuCID0013841 Assay Type SYBR® Green Detected Coding Transcript(s) ENSMUST00000022535 Amplicon Context Sequence TAATCTGGGAAGCACCGAGACTCTAGAAGAGACACCCTCTGGGTCACAGGATAA GTCTGCTCCGTCTGGTCATAAACATCTGACAGTAGAAGAGTTATTTGGAACCTCC TTGCCAAAGGAA Amplicon Length (bp) 91 Chromosome Location 14:30513043-30518984 Assay Design Intron-spanning Purification Desalted Validation Results Efficiency (%) 98 R2 0.9997 cDNA Cq 22.41 cDNA Tm (Celsius) 81 gDNA Cq 24.87 Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 1/4 PrimePCR™Assay Validation Report Dcp1a, Mouse Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak Melt curve analysis of above amplification Standard Curve Standard curve generated using 20 million copies of template diluted 10-fold to 20 copies Page 2/4 PrimePCR™Assay Validation Report Products used to generate validation data Real-Time PCR Instrument CFX384 Real-Time PCR Detection System Reverse Transcription Reagent iScript™ Advanced cDNA Synthesis Kit for RT-qPCR Real-Time PCR Supermix SsoAdvanced™ SYBR® Green Supermix Experimental Sample qPCR Mouse Reference Total RNA Data Interpretation Unique Assay ID This is a unique identifier that can be used to identify the assay in the literature and online. Detected Coding Transcript(s) This is a list of the Ensembl transcript ID(s) that this assay will detect. -

Exon Amplification: a Strategy to Isolate Mammalian Genes Based on RNA Splicing (Gene Cloning/Polymerase Chain Reaction) ALAN J
Proc. Natl. Acad. Sci. USA Vol. 88, pp. 4005-4009, May 1991 Genetics Exon amplification: A strategy to isolate mammalian genes based on RNA splicing (gene cloning/polymerase chain reaction) ALAN J. BUCKLER*, DAVID D. CHANG, SHARON L. GRAw, J. DAVID BROOK, DANIEL A. HABER, PHILLIP A. SHARP, AND DAVID E. HOUSMAN Center for Cancer Research, Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139 Contributed by Phillip A. Sharp, January 25, 1991 ABSTRACT We have developed a method, exon amplifi- (7, 8). Thus, this method may be generally applicable for the cation, for fast and efficient isolation of coding sequences from selection of exon sequences from any gene. The method is complex mammalian genomic DNA. This method is based on also both rapid and easily adapted to large scale experiments. the selection of RNA sequences, exons, which are flanked by A series of cloned genomic DNA fragments can be screened functional 5' and 3' splice sites. Fragments of cloned genomic within 1-2 weeks. The sensitivity of this method is high. DNA are inserted into an intron, which is flanked by 5' and 3' Genomic DNA segments of 20 kilobases (kb) or more can be splice sites of the human immunodeficiency virus 1 tat gene successfully screened in a single transfection by using a set contained within the plasmid pSPL1. COS-7 cells are trans- of pooled subclones. This method thus allows the rapid fected with these constructs, and the resulting RNA transcripts identification of exons in mammalian genomic DNA and are processed in vivo. Splice sites of exons contained within the should facilitate the isolation of a wide spectrum of genes of inserted genomic fragment are paired with splice sites of the significance in physiology and development. -

Mrna Turnover Philip Mitchell* and David Tollervey†
320 mRNA turnover Philip Mitchell* and David Tollervey† Nuclear RNA-binding proteins can record pre-mRNA are cotransported to the cytoplasm with the mRNP. These processing events in the structure of messenger proteins may preserve a record of the nuclear history of the ribonucleoprotein particles (mRNPs). During initial rounds of pre-mRNA in the cytoplasmic mRNP structure. This infor- translation, the mature mRNP structure is established and is mation can strongly influence the cytoplasmic fate of the monitored by mRNA surveillance systems. Competition for the mRNA and is used by mRNA surveillance systems that act cap structure links translation and subsequent mRNA as a checkpoint of mRNP integrity, particularly in the identi- degradation, which may also involve multiple deadenylases. fication of premature translation termination codons (PTCs). Addresses Cotransport of nuclear mRNA-binding proteins with mRNA Wellcome Trust Centre for Cell Biology, ICMB, University of Edinburgh, from the nucleus to the cytoplasm (nucleocytoplasmic shut- Kings’ Buildings, Edinburgh EH9 3JR, UK tling) was first observed for the heterogeneous nuclear *e-mail: [email protected] ribonucleoprotein (hnRNP) proteins. Some hnRNP proteins †e-mail: [email protected] are stripped from the mRNA at export [1], but hnRNP A1, Current Opinion in Cell Biology 2001, 13:320–325 A2, E, I and K are all exported (see [2]). Although roles for 0955-0674/01/$ — see front matter these hnRNP proteins in transport and translation have been © 2001 Elsevier Science Ltd. All rights reserved. reported [3•,4•], their affects on mRNA stability have been little studied. More is known about hnRNP D/AUF1 and Abbreviations AREs AU-rich sequence elements another nuclear RNA-binding protein, HuR, which act CBC cap-binding complex antagonistically to modulate the stability of a range of DAN deadenylating nuclease mRNAs containing AU-rich sequence elements (AREs) DSEs downstream sequence elements (reviewed in [2]). -

Supplemental Information
Supplemental information Dissection of the genomic structure of the miR-183/96/182 gene. Previously, we showed that the miR-183/96/182 cluster is an intergenic miRNA cluster, located in a ~60-kb interval between the genes encoding nuclear respiratory factor-1 (Nrf1) and ubiquitin-conjugating enzyme E2H (Ube2h) on mouse chr6qA3.3 (1). To start to uncover the genomic structure of the miR- 183/96/182 gene, we first studied genomic features around miR-183/96/182 in the UCSC genome browser (http://genome.UCSC.edu/), and identified two CpG islands 3.4-6.5 kb 5’ of pre-miR-183, the most 5’ miRNA of the cluster (Fig. 1A; Fig. S1 and Seq. S1). A cDNA clone, AK044220, located at 3.2-4.6 kb 5’ to pre-miR-183, encompasses the second CpG island (Fig. 1A; Fig. S1). We hypothesized that this cDNA clone was derived from 5’ exon(s) of the primary transcript of the miR-183/96/182 gene, as CpG islands are often associated with promoters (2). Supporting this hypothesis, multiple expressed sequences detected by gene-trap clones, including clone D016D06 (3, 4), were co-localized with the cDNA clone AK044220 (Fig. 1A; Fig. S1). Clone D016D06, deposited by the German GeneTrap Consortium (GGTC) (http://tikus.gsf.de) (3, 4), was derived from insertion of a retroviral construct, rFlpROSAβgeo in 129S2 ES cells (Fig. 1A and C). The rFlpROSAβgeo construct carries a promoterless reporter gene, the β−geo cassette - an in-frame fusion of the β-galactosidase and neomycin resistance (Neor) gene (5), with a splicing acceptor (SA) immediately upstream, and a polyA signal downstream of the β−geo cassette (Fig. -

Sequences at the Exon-Intron Boundaries* (Split Gene/Mrna Splicing/Eukaryotic Gene Structure) R
Proc. Nati. Acad. Sci. USA Vol. 75, No. 10, pp. 4853-4857, October 1978 Biochemistry Ovalbumin gene: Evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries* (split gene/mRNA splicing/eukaryotic gene structure) R. BREATHNACH, C. BENOIST, K. O'HARE, F. GANNON, AND P. CHAMBON Laboratoire de Genetique Mol6culaire des Eucaryotes du Centre National de la Recherche Scientifique, Unite 44 de l'Institut National de la Sant6 et de la Recherche MWdicale, Institut de Chimie Biologique, Facult6 de Melecine, Strasbourg 67085, France Communicated by A. Frey-Wyssling, July 31, 1978 ABSTRACT Selected regions of cloned EcoRI fragments the 5' end of ov-mRNA and have revealed some interesting of the chicken ovalbumin gene have been sequenced. The po- features in the DNA sequences at exon-intron boundaries. sitions where the sequences coding for ovalbumin mRNA (ov- mRNA) are interrupted in the genome have been determined, and a previously unreported interruption in the DNA sequences MATERIALS AND METHODS coding for the 5' nontranslated region of the messenger has been discovered. Because directly repeated sequences are found at Plasmid pCR1 ov 2.1 containing the ov-ds-cDNA insert was exon-intron boundaries, the nucleotide sequence alone cannot prepared as described (9). EcoRI fragments "b," "c," and "d" define unique excision-ligation points for the processing of a previously cloned in X vectors (3) were transferred to the plas- possible ov-mRNA precursor. However, the sequences in these mid pBR 322. An EcoRI/HindIII of the EcoRI boundary regions share common features; this leads to the fragment proposal that there are, in fact, unique excision-ligation points fragment "a" containing the entirety of exon 7 (Fig. -

Nuclear PTEN Safeguards Pre-Mrna Splicing to Link Golgi Apparatus for Its Tumor Suppressive Role
ARTICLE DOI: 10.1038/s41467-018-04760-1 OPEN Nuclear PTEN safeguards pre-mRNA splicing to link Golgi apparatus for its tumor suppressive role Shao-Ming Shen1, Yan Ji2, Cheng Zhang1, Shuang-Shu Dong2, Shuo Yang1, Zhong Xiong1, Meng-Kai Ge1, Yun Yu1, Li Xia1, Meng Guo1, Jin-Ke Cheng3, Jun-Ling Liu1,3, Jian-Xiu Yu1,3 & Guo-Qiang Chen1 Dysregulation of pre-mRNA alternative splicing (AS) is closely associated with cancers. However, the relationships between the AS and classic oncogenes/tumor suppressors are 1234567890():,; largely unknown. Here we show that the deletion of tumor suppressor PTEN alters pre-mRNA splicing in a phosphatase-independent manner, and identify 262 PTEN-regulated AS events in 293T cells by RNA sequencing, which are associated with significant worse outcome of cancer patients. Based on these findings, we report that nuclear PTEN interacts with the splicing machinery, spliceosome, to regulate its assembly and pre-mRNA splicing. We also identify a new exon 2b in GOLGA2 transcript and the exon exclusion contributes to PTEN knockdown-induced tumorigenesis by promoting dramatic Golgi extension and secretion, and PTEN depletion significantly sensitizes cancer cells to secretion inhibitors brefeldin A and golgicide A. Our results suggest that Golgi secretion inhibitors alone or in combination with PI3K/Akt kinase inhibitors may be therapeutically useful for PTEN-deficient cancers. 1 Department of Pathophysiology, Key Laboratory of Cell Differentiation and Apoptosis of Chinese Ministry of Education, Shanghai Jiao Tong University School of Medicine (SJTU-SM), Shanghai 200025, China. 2 Institute of Health Sciences, Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences and SJTU-SM, Shanghai 200025, China. -

The Lifespan of an MLL-Rearranged Therapy-Related Paediatric AML
Bone Marrow Transplantation (2015) 50, 1382–1384 © 2015 Macmillan Publishers Limited All rights reserved 0268-3369/15 www.nature.com/bmt LETTER TO THE EDITOR From initiation to eradication: the lifespan of an MLL-rearranged therapy-related paediatric AML Bone Marrow Transplantation (2015) 50, 1382–1384; doi:10.1038/ amplified by RT-PCR. DCP1A is predominantly cytoplasmic as part bmt.2015.155; published online 6 July 2015 of a protein complex involved in degradation of mRNAs.8 Upon stimulation by TGFB1 and interaction with SMAD4, it becomes nuclear contributing to the transactivation of TGF-α target genes.9 Therapy-related AML (tAML) is one of the most prevalent DCP1A is a novel MLL fusion partner in AML. The in vitro secondary malignant neoplasms in paediatric cancer survivors. transforming capacity of MLL-DCP1A was weaker than the more Estimates of incidence and association with causative chemo- common MLL-ENL fusion but it could nevertheless be clearly therapeutic agents are mainly based on epidemiological data. verified by colony formation in retroviral transduction-replating 10 Individual courses revealing the exact timing of the first clone assays (Figure 1d). appearance and its development under treatment are limited The cumulative sampling of bone marrow during preceding owing to the overall low incidence, which does not justify neuroblastoma treatment offered the unique possibility to repeated bone marrow sampling in order to detect the occurrence quantitatively backtrack the origin of the tAML by quantitative of potentially leukemic cells. real-time PCR using the genomic MLL-DCP1A breakpoint as a Here we report a unique case of tAML arising after neuro- molecular marker. -

Spliceosomal Intronogenesis
Spliceosomal intronogenesis Sujin Leea and Scott W. Stevensb,c,1 aGraduate Program in Cellular and Molecular Biology, The University of Texas at Austin, Austin, TX 78712; bDepartment of Molecular Biosciences, The University of Texas at Austin, Austin, TX 78712; and cInstitute for Cellular and Molecular Biology, The University of Texas at Austin, Austin, TX 78712 Edited by Jef D. Boeke, New York University School of Medicine, New York, NY, and approved April 20, 2016 (received for review March 30, 2016) The presence of intervening sequences, termed introns, is a defining it is clear that introns massively infiltrated the genome of the last characteristic of eukaryotic nuclear genomes. Once transcribed into eukaryotic common ancestor and that introns have continued to be pre-mRNA, these introns must be removed within the spliceosome gained and lost over evolutionary time (11, 18). before export of the processed mRNA to the cytoplasm, where it is Here we report the use of a reporter system designed to detect translated into protein. Although intron loss has been demonstrated events of intron gain and intron loss in the budding yeast Sac- experimentally, several mysteries remain regarding the origin and charomyces cerevisiae. With this reporter, we have captured, to our propagation of introns. Indeed, documented evidence of gain of an knowledge, the first verified examples of intron gain via intron intron has only been suggested by phylogenetic analyses. We report transposition in any eukaryote. the use of a strategy that detects selected intron gain and loss events. We have experimentally verified, to our knowledge, the first demon- Results strations of intron transposition in any organism. -

RNA Splicing: Unexpected Spliceosome Diversity Jan-Peter Kreivi and Angus I
View metadata, citation and similar papers at core.ac.uk brought to you by CORE 802 Dispatch provided by Elsevier - Publisher Connector RNA splicing: Unexpected spliceosome diversity Jan-Peter Kreivi and Angus I. Lamond A novel form of spliceosome, containing the minor Figure 1 snRNPs U11 and U12, splices a class of pre-mRNA introns with non-consensus splice sites. This unexpected GT–AG intron spliceosome diversity has interesting implications for the evolution and expression of eukaryotic genes. * AG GTRAGT TNCTRAC Py CAG G Address: Department of Biochemistry, University of Dundee, Dundee, 5' splice site Branch site 3' splice site DD1 4HN, Scotland, UK. Current Biology 1996, Vol 6 No 7:802–805 AT–AC intron © Current Biology Ltd ISSN 0960-9822 * ATATCCTT TCCTTAAC YCCAC The discovery that most protein-coding genes in eukary- 5' splice site Branch site 3' splice site otes are interrupted by one or more intervening sequences, or introns, was one of the major advances in © 1996 Current Biology molecular biology made in the 1970s [1,2]. The formation of mRNA thus involves a splicing process to remove Conserved elements in mammalian GT–AG and AT–AC introns. The introns from primary gene transcripts. Splicing takes first and last two nucleotides of the introns are shown in bold and the branch-site adenosine residue that forms the lariat structure is marked place in the nucleus before the mRNAs are exported to by an asterisk. The polypyrimidine tract that lies between the branch the cytoplasm for translation and occurs by a two-step site and 3′ intron–exon junction of GT–AG introns is lacking in AT–AC mechanism, in which both steps involve transesterifica- introns. -

Aspli: an Integrative R Package for Analysing Alternative Splicing Using RNA-Seq
ASpli: An integrative R package for analysing alternative splicing using RNA-seq Estefania Mancini, Andres Rabinovich, Javier Iserte, Marcelo Yanovsky, Ariel Chernomoretz May 19, 2021 Contents 1 Introduction ..............................2 2 Citation.................................2 3 Quick start ...............................3 4 Getting started ............................5 4.1 Installation ............................5 4.2 Required input data........................5 5 ASpli overview ............................6 6 Using ASpli ..............................7 6.1 binGenome: Binning the genome ..................8 6.1.1 Bin definition.......................9 6.1.2 Splicing event assignment..................9 6.2 Read counting .......................... 11 6.2.1 Targets data.frame definition................. 11 6.2.2 gbCounts: Summarize read overlaps against all feature levels.. 11 6.2.3 jCounts: Summarize junctions inclussion indices PSI, PIR and PJU........................... 13 6.3 Differential signals ........................ 16 6.3.1 gbDUreport: Bin-based coverage differential signals of AS.... 16 6.3.2 jDUreport: Junction-centered analysis of AS.......... 18 6.4 Integrative reports ........................ 20 6.4.1 splicingReport: bin and junction signals integration...... 20 6.4.2 integrateSignals(): Region specific summarization of differential usage signals........................ 20 6.4.3 exportSplicingReport: Export splicing reports in HTML pages... 24 6.4.4 exportIntegratedSignals(): Export integrated signals into HTML pages..........................