Universityof Cape Town
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arctic Expedition12° 16° 20° 24° 28° 32° Spitsbergen U Svalbard Archipelago 80° 80°
distinguished travel for more than 35 years Voyage UNDER THE Midnight Sun Arctic Expedition12° 16° 20° 24° 28° 32° Spitsbergen u Svalbard Archipelago 80° 80° 80° Raudfjorden Nordaustlandet Woodfjorden Smeerenburg Monaco Glacier The Arctic’s 79° 79° 79° Kongsfjorden Svalbard King’s Glacier Archipelago Ny-Ålesund Spitsbergen Longyearbyen Canada 78° 78° 78° i Greenland tic C rcle rc Sea Camp Millar A U.S. North Pole Russia Bellsund Calypsobyen Svalbard Archipelago Norway Copenhagen Burgerbukta 77° 77° 77° Cruise Itinerary Denmark Air Routing Samarin Glacier Hornsund Barents Sea June 20 to 30, 2022 4° 8° Spitsbergen12° u Samarin16° Glacier20° u Calypsobyen24° 76° 28° 32° 36° 76° Voyage across the Arctic Circle on this unique 11-day Monaco Glacier u Smeerenburg u Ny-Ålesund itinerary featuring a seven-night cruise round trip Copenhagen 1 Depart the U.S. or Canada aboard the Five-Star Le Boréal. Visit during the most 2 Arrive in Copenhagen, Denmark enchanting season, when the region is bathed in the magical 3 Copenhagen/Fly to Longyearbyen, Spitsbergen, light of the Midnight Sun. Cruise the shores of secluded Norway’s Svalbard Archipelago/Embark Le Boréal 4 Hornsund for Burgerbukta/Samarin Glacier Spitsbergen—the jewel of Norway’s rarely visited Svalbard 5 Bellsund for Calypsobyen/Camp Millar archipelago enjoy expert-led Zodiac excursions through 6 Cruising the Arctic Ice Pack sandstone mountain ranges, verdant tundra and awe-inspiring 7 MåkeØyane/Woodfjorden/Monaco Glacier ice formations. See glaciers calve in luminous blues and search 8 Raudfjorden for Smeerenburg for Arctic wildlife, including the “King of the Arctic,” the 9 Ny-Ålesund/Kongsfjorden for King’s Glacier polar bear, whales, walruses and Svalbard reindeer. -

Freshwater Fishes
WESTERN CAPE PROVINCE state oF BIODIVERSITY 2007 TABLE OF CONTENTS Chapter 1 Introduction 2 Chapter 2 Methods 17 Chapter 3 Freshwater fishes 18 Chapter 4 Amphibians 36 Chapter 5 Reptiles 55 Chapter 6 Mammals 75 Chapter 7 Avifauna 89 Chapter 8 Flora & Vegetation 112 Chapter 9 Land and Protected Areas 139 Chapter 10 Status of River Health 159 Cover page photographs by Andrew Turner (CapeNature), Roger Bills (SAIAB) & Wicus Leeuwner. ISBN 978-0-620-39289-1 SCIENTIFIC SERVICES 2 Western Cape Province State of Biodiversity 2007 CHAPTER 1 INTRODUCTION Andrew Turner [email protected] 1 “We live at a historic moment, a time in which the world’s biological diversity is being rapidly destroyed. The present geological period has more species than any other, yet the current rate of extinction of species is greater now than at any time in the past. Ecosystems and communities are being degraded and destroyed, and species are being driven to extinction. The species that persist are losing genetic variation as the number of individuals in populations shrinks, unique populations and subspecies are destroyed, and remaining populations become increasingly isolated from one another. The cause of this loss of biological diversity at all levels is the range of human activity that alters and destroys natural habitats to suit human needs.” (Primack, 2002). CapeNature launched its State of Biodiversity Programme (SoBP) to assess and monitor the state of biodiversity in the Western Cape in 1999. This programme delivered its first report in 2002 and these reports are updated every five years. The current report (2007) reports on the changes to the state of vertebrate biodiversity and land under conservation usage. -

Climate in Svalbard 2100
M-1242 | 2018 Climate in Svalbard 2100 – a knowledge base for climate adaptation NCCS report no. 1/2019 Photo: Ketil Isaksen, MET Norway Editors I.Hanssen-Bauer, E.J.Førland, H.Hisdal, S.Mayer, A.B.Sandø, A.Sorteberg CLIMATE IN SVALBARD 2100 CLIMATE IN SVALBARD 2100 Commissioned by Title: Date Climate in Svalbard 2100 January 2019 – a knowledge base for climate adaptation ISSN nr. Rapport nr. 2387-3027 1/2019 Authors Classification Editors: I.Hanssen-Bauer1,12, E.J.Førland1,12, H.Hisdal2,12, Free S.Mayer3,12,13, A.B.Sandø5,13, A.Sorteberg4,13 Clients Authors: M.Adakudlu3,13, J.Andresen2, J.Bakke4,13, S.Beldring2,12, R.Benestad1, W. Bilt4,13, J.Bogen2, C.Borstad6, Norwegian Environment Agency (Miljødirektoratet) K.Breili9, Ø.Breivik1,4, K.Y.Børsheim5,13, H.H.Christiansen6, A.Dobler1, R.Engeset2, R.Frauenfelder7, S.Gerland10, H.M.Gjelten1, J.Gundersen2, K.Isaksen1,12, C.Jaedicke7, H.Kierulf9, J.Kohler10, H.Li2,12, J.Lutz1,12, K.Melvold2,12, Client’s reference 1,12 4,6 2,12 5,8,13 A.Mezghani , F.Nilsen , I.B.Nilsen , J.E.Ø.Nilsen , http://www.miljodirektoratet.no/M1242 O. Pavlova10, O.Ravndal9, B.Risebrobakken3,13, T.Saloranta2, S.Sandven6,8,13, T.V.Schuler6,11, M.J.R.Simpson9, M.Skogen5,13, L.H.Smedsrud4,6,13, M.Sund2, D. Vikhamar-Schuler1,2,12, S.Westermann11, W.K.Wong2,12 Affiliations: See Acknowledgements! Abstract The Norwegian Centre for Climate Services (NCCS) is collaboration between the Norwegian Meteorological In- This report was commissioned by the Norwegian Environment Agency in order to provide basic information for use stitute, the Norwegian Water Resources and Energy Directorate, Norwegian Research Centre and the Bjerknes in climate change adaptation in Svalbard. -

Arctic Environments
Characteristics of an arctic environment and the physical geography of Svalbard - ‘geography explained’ fact sheet The Arctic environment is little studied at Key Stage Three yet it is an excellent basis for an all-encompassing study of place or as a case study to illustrate key concepts within a specific theme. Svalbard, an archipelago lying in the Arctic Ocean north of mainland Europe, about midway between Norway and the North Pole, is a place with an awesome landscape and unique geography that includes issues and themes of global, regional and local importance. A study of Svalbard could allow pupils to broaden and deepen their knowledge and understanding of different aspects of the seven geographical concepts that underpin the revised Geography Key Stage Three Programme of Study. Many pupils will have a mental image of an Arctic landscape, some may have heard of Svalbard. A useful starting point for study is to explore these perceptions using visual prompts and big questions – where is the Arctic/Svalbard? What is it like? What is happening there? Why is it like this? How will it change? Svalbard exemplifies the distinctive physical and human characteristics of the Arctic and yet is also unique amongst Arctic environments. Perceptions and characteristics of the Arctic may be represented in many ways, including art and literature and the pupil’s own geographical imagination of the place. Maps and photographs are vital in helping pupils develop spatial understanding of locations, places and processes and the scale at which they occur. Source: commons.wikimedia.org/wiki/Image:W_W_Svalbard... 1 Longyearbyen, Svalbard’s capital Source:http://www.photos- The landscape of Western Svalbard voyages.com/spitzberg/images/spitzberg06_large.jpg Source: www.hi.is/~oi/svalbard_photos.htm Where is Svalbard? Orthographic map projection centred on Svalbard and showing location relative to UK and EuropeSource: www.answers.com/topic/orthographic- projection.. -

Youth Guide to Biodiversity
SPECIES ECOSYSTEMS HABITATS SOILS CLOUDS LAKES RIVERS MOUNTAINS HUMANS INSECTS MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES ACTIONS HABITS ORGANIZATIONS SPECIES ECOSYSTEMS HABITATS SOILS CLOUDS LAKES RIVERS MOUNTAINS HUMANS INSECTS MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES ACTIONS HABITS ORGANIZATIONS SPECIES ECOSYSTEMS HABITATS SOILS CLOUDS LAKES RIVERS MOUNTAINS HUMANS INSECTS YOUTH AND UNITED NATIONS GLOBAL ALLIANCE LEARNING AND ACTION SERIES MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES ACTIONS HABITS ORGANIZATIONS SPECIES ECOSYSTEMS HABITATS SOILS CLOUDS LAKES RIVERS YOUTH AND UNITED NATIONS GLOBAL ALLIANCE LEARNING AND ACTION SERIES SUPPORTED BY MOUNTAINS HUMANS INSECTS MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES ACTIONS HABITS ORGANIZATIONS SPECIES ECOSYSTEMS HABITATS TAKE ACTION FOR BIODIVERSITY! THE TO SOILS CLOUDS LAKES RIVERS MOUNTAINS HUMANS INSECTS MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES ACTIONS HABITS ORGANIZATIONS SPECIES ECOSYSTEMS HABITATS SOILS CLOUDS LAKES RIVERS MOUNTAINS HUMANS INSECTS MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES YOUTHGUIDE BIODIVERSITY ACTIONS HABITSWE ORGANIZATIONS ARE SPECIES ECOSYSTEMS MANY HABITATS SOILS CLOUDS LAKES RIVERS MOUNTAINS HUMANS INSECTS MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES ACTIONS HABITS ORGANIZATIONS SPECIES ECOSYSTEMS HABITATS SOILS CLOUDS LAKES RIVERS MOUNTAINS HUMANS INSECTS MAMMALS OCEANS BIRDS FISHES WORMS PLANTS FLOWERS BEES ACTIONS HABITS ORGANIZATIONSwww. SPECIES ECOSYSTEMS HABITATS SOILS CLOUDS -

Constraints on the Timescale of Animal Evolutionary History
Palaeontologia Electronica palaeo-electronica.org Constraints on the timescale of animal evolutionary history Michael J. Benton, Philip C.J. Donoghue, Robert J. Asher, Matt Friedman, Thomas J. Near, and Jakob Vinther ABSTRACT Dating the tree of life is a core endeavor in evolutionary biology. Rates of evolution are fundamental to nearly every evolutionary model and process. Rates need dates. There is much debate on the most appropriate and reasonable ways in which to date the tree of life, and recent work has highlighted some confusions and complexities that can be avoided. Whether phylogenetic trees are dated after they have been estab- lished, or as part of the process of tree finding, practitioners need to know which cali- brations to use. We emphasize the importance of identifying crown (not stem) fossils, levels of confidence in their attribution to the crown, current chronostratigraphic preci- sion, the primacy of the host geological formation and asymmetric confidence intervals. Here we present calibrations for 88 key nodes across the phylogeny of animals, rang- ing from the root of Metazoa to the last common ancestor of Homo sapiens. Close attention to detail is constantly required: for example, the classic bird-mammal date (base of crown Amniota) has often been given as 310-315 Ma; the 2014 international time scale indicates a minimum age of 318 Ma. Michael J. Benton. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Philip C.J. Donoghue. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Robert J. -
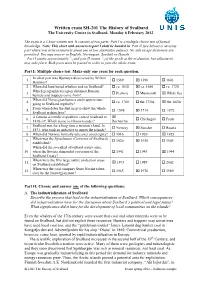
Written Exam SH-201 the History of Svalbard the University Centre in Svalbard, Monday 6 February 2012
Written exam SH-201 The History of Svalbard The University Centre in Svalbard, Monday 6 February 2012 The exam is a 3 hour written test. It consists of two parts: Part I is a multiple choice test of factual knowledge. Note: This sheet with answers to part I shall be handed in. Part II (see below) is an essay part where you write extensively about one of two alternative subjects. No aids except dictionary are permitted. You may answer in English, Norwegian, Swedish or Danish. 1 2 Part I counts approximately /3 and part II counts /3 of the grade at the evaluation, but adjustment may take place. Both parts must be passed in order to pass the whole exam. Part I: Multiple choice test. Make only one cross for each question. In what year was Bjørnøya discovered by Willem 1. 1569 1596 1603 Barentsz? 2. When did land-based whaling end on Svalbard? ca. 1630 ca. 1680 ca. 1720 Which geographical region did most Russian 3. Pechora Murmansk White Sea hunters and trappers come from? When did Norwegian hunters and trappers start 4. ca. 1700 the 1750s the 1820s going to Svalbard regularly? From when dates the first map to show the whole 5. 1598 1714 1872 Svalbard archipelago? A famous scientific expedition visited Svalbard in 6. Chichagov Fram 1838–39. Which name is it known under? Recherche Svalbard was for a long time a no man’s land. In 7. Norway Sweden Russia 1871, who took an initiative to annex the islands? 8. When did Norway formally take over sovereignty? 1916 1920 1925 When was the Sysselmann (Governor of Svalbard) 9. -

AMNH Digital Library
"^^ Ji\!*"^ ^i r ^ THE GREATEST RISK IS NOT TAKING ONE. for years? What does it take to break through a foundation of thinking that's been accepted all A bold stroke. But, it doesn't mean turning things on their heads without understanding the possible consequences, it takes a complete understanding of what lies on the other side. No one is in a better position to help guide clients than AiG. With operations in 130 countries and jurisdictions throughout the world, we have an unmatched knowledge of local conditions and regulations to help manage risks. So, when you're ready to take on a risky venture, contact AIG. We'll help knock down the barriers that may be holding you back. WORLD LEADERS IN INSURANCE AND FINANCIAL SERVICES AIG Insurance and services provided by members of American International Group. Inc.. 70 Pine Street, Department A, New York. New York 10270. With Bristol-Myers Squibb research, , a tiny pair of chromosomes could make a huge difference to this pair of Armstrongs. W" 4^ ^>i, '•^l ^ii^S^BKmKEt ^^W«» mmm^m^^' ";'*•" mmmmam m;s!J5i-i'iw^.^ "''^''. Three-time Tour de France winner ^i''^v% • Lance Armstrong, with his son Luke. gj^^2 ^'^^^^^^^^'^^-i'^^^S!^iSK jgmLiJmL Five years ago, cyclist Lance Armstrong was diagnosed with testicular cancer. It had already spread to his abdomen, lungs and brain. But with aggressive treatments, including three Bristol-Myers Squibb medicines. Lance conquered his cancer. He has gone on to win the Tour de France three times, and even to have a wonderful son— Luke. -

Australia's Biodiversity and Climate Change
Australia’s Biodiversity and Climate Change A strategic assessment of the vulnerability of Australia’s biodiversity to climate change A report to the Natural Resource Management Ministerial Council commissioned by the Australian Government. Prepared by the Biodiversity and Climate Change Expert Advisory Group: Will Steffen, Andrew A Burbidge, Lesley Hughes, Roger Kitching, David Lindenmayer, Warren Musgrave, Mark Stafford Smith and Patricia A Werner © Commonwealth of Australia 2009 ISBN 978-1-921298-67-7 Published in pre-publication form as a non-printable PDF at www.climatechange.gov.au by the Department of Climate Change. It will be published in hard copy by CSIRO publishing. For more information please email [email protected] This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the Commonwealth. Requests and inquiries concerning reproduction and rights should be addressed to the: Commonwealth Copyright Administration Attorney-General's Department 3-5 National Circuit BARTON ACT 2600 Email: [email protected] Or online at: http://www.ag.gov.au Disclaimer The views and opinions expressed in this publication are those of the authors and do not necessarily reflect those of the Australian Government or the Minister for Climate Change and Water and the Minister for the Environment, Heritage and the Arts. Citation The book should be cited as: Steffen W, Burbidge AA, Hughes L, Kitching R, Lindenmayer D, Musgrave W, Stafford Smith M and Werner PA (2009) Australia’s biodiversity and climate change: a strategic assessment of the vulnerability of Australia’s biodiversity to climate change. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

Bradypodion Caeruleogula, Eshowe Dwarf Chameleon
The IUCN Red List of Threatened Species™ ISSN 2307-8235 (online) IUCN 2008: T172551A110305774 Scope: Global Language: English Bradypodion caeruleogula, Eshowe Dwarf Chameleon Assessment by: Tolley, K. View on www.iucnredlist.org Citation: Tolley, K. 2017. Bradypodion caeruleogula. The IUCN Red List of Threatened Species 2017: e.T172551A110305774. http://dx.doi.org/10.2305/IUCN.UK.2017-1.RLTS.T172551A110305774.en Copyright: © 2017 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale, reposting or other commercial purposes is prohibited without prior written permission from the copyright holder. For further details see Terms of Use. The IUCN Red List of Threatened Species™ is produced and managed by the IUCN Global Species Programme, the IUCN Species Survival Commission (SSC) and The IUCN Red List Partnership. The IUCN Red List Partners are: Arizona State University; BirdLife International; Botanic Gardens Conservation International; Conservation International; NatureServe; Royal Botanic Gardens, Kew; Sapienza University of Rome; Texas A&M University; and Zoological Society of London. If you see any errors or have any questions or suggestions on what is shown in this document, please provide us with feedback so that we can correct or extend the information provided. THE IUCN RED LIST OF THREATENED SPECIES™ Taxonomy Kingdom Phylum Class Order Family Animalia Chordata Reptilia Squamata Chamaeleonidae Taxon Name: Bradypodion caeruleogula Raw & Brothers, 2008 Common Name(s): • English: Eshowe Dwarf Chameleon, Dhlinza Dwarf Chameleon, uMlalazi Dwarf Chameleon Taxonomic Notes: Recently described from Dlinza Forest (Raw and Brothers 2008). -

MARITIME ACTIVITY in the HIGH NORTH – CURRENT and ESTIMATED LEVEL up to 2025 MARPART Project Report 1
MARITIME ACTIVITY IN THE HIGH NORTH – CURRENT AND ESTIMATED LEVEL UP TO 2025 MARPART Project Report 1 Authors: Odd Jarl Borch, Natalia Andreassen, Nataly Marchenko, Valur Ingimundarson, Halla Gunnarsdóttir, Iurii Iudin, Sergey Petrov, Uffe Jacobsen and Birita í Dali List of authors Odd Jarl Borch Project Leader, Nord University, Norway Natalia Andreassen Nord University, Norway Nataly Marchenko The University Centre in Svalbard, Norway Valur Ingimundarson University of Iceland Halla Gunnarsdóttir University of Iceland Iurii Iudin Murmansk State Technical University, Russia Sergey Petrov Murmansk State Technical University, Russia Uffe Jakobsen University of Copenhagen, Denmark Birita í Dali University of Greenland 1 Partners MARPART Work Package 1 “Maritime Activity and Risk” 2 THE MARPART RESEARCH CONSORTIUM The management, organization and governance of cross-border collaboration within maritime safety and security operations in the High North The key purpose of this research consortium is to assess the risk of the increased maritime activity in the High North and the challenges this increase may represent for the preparedness institutions in this region. We focus on cross-institutional and cross-country partnerships between preparedness institutions and companies. We elaborate on the operational crisis management of joint emergency operations including several parts of the preparedness system and resources from several countries. The project goals are: • To increase understanding of the future demands for preparedness systems in the High North including both search and rescue, oil spill recovery, fire fighting and salvage, as well as capacities fighting terror or other forms of destructive action. • To study partnerships and coordination challenges related to cross-border, multi-task emergency cooperation • To contribute with organizational tools for crisis management Project characteristics: Financial support: -Norwegian Ministry of Foreign Affairs, -the Nordland county Administration -University partners.