Directions for Use Tandemlung Oxygenator CAT# 5160-0000
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

8 Implantable Membrane Oxygenator
Artificial Lung 8 . Cardiopulmonary Bypass - oxgenator 1 Cardiopulmonary Bypass § Cardiopulmonary bypass (CPB), also called heart-lung bypass, allows the temporary replacement of the gas exchange function of the lungs and the blood-pumping function of the heart § The terms pump-oxygenator and heart–lung machine graphically describe the equipment used § By extension, the term is also applies to surgical procedures which take place primarily on the external aspects of the heart, such as creating new routes for blood to reach the distal coronary arteries from the aorta 3 Cardiopulmonary Bypass § As usually employed for cardiac surgery, the heart–lung machine is part of a total, venoarterial cardiopulmonary bypass circuit à meaning that all the venous blood returning to the right heart cavities is collected in the extracorporeal circuit à circulated through the gas exchange device, from where it is pumped back into the arterial tree à thereby “bypassing” the heart cavities and the pulmonary circulation 5 Cardiopulmonary Bypass Scheme of standard operating conditions during cardiopulmonary bypass 6 Cardiopulmonary Bypass 7 Artificial Lung Artificial Lung vs. Natural Lung 8 Artificial Lung vs. Natural Lung § In the natural lungs, the factors underlying exchange across the alveolo-capillary barrier and transport by the blood can be grouped into four classes:- ú The ventilation of the lungs (the volume flow rate of gas) and the composition of the gas mixture to which mixed venous (pulmonary artery) blood will be exposed 9 Artificial Lung vs. Natural Lung § In the natural lungs, the factors underlying exchange across the alveolo-capillary barrier and transport by the blood can be grouped into four classes:- ú The permeability of the materials which separate the gas phase from the blood phase in the pulmonary alveoli 10 Artificial Lung vs. -

Left Atrial to Femoral Artery Full Cardiopulmonary Bypass: a Novel Technique for Descending and Thoracoabdominal Aortic Surgery
Published online: 2019-12-09 Original Article 19 Left Atrial to Femoral Artery Full Cardiopulmonary Bypass: A Novel Technique for Descending and Thoracoabdominal Aortic Surgery Dimitra Papanikolaou, MD1 Chris Savio, BS, CCP1 Mohammad A. Zafar, MBBS1 Leon Freudzon, MD1 Jinlin Wu, MD1 Mohamed Abdelbaky, MD1 Keith J. Pelletier, MBA, MHS, CCP1 Joelle Buntin, MSN, RN, RN-BC1 Thais Faggion Vinholo, BS, MSc1 Bulat A. Ziganshin, MD, PhD1,2 Brian Schwartz, MS, CCP1 John A. Elefteriades, MD, PhD (Hon)1 1 Aortic Institute at Yale-New Haven Hospital, Yale University School Address for correspondence John A. Elefteriades, MD, Aortic Institute of Medicine, New Haven, Connecticut at Yale-New Haven, Yale University School of Medicine, Clinic Building 2 Department of Surgical Diseases, Kazan State Medical University, CB 317, 789 Howard Avenue, New Haven, CT 06519 Kazan, Russia (e-mail: [email protected]). Int J Angiol 2020;29:19–26. Abstract Left atrial-femoral artery (LA-FA) bypass with a centrifugal pump and no oxygenator is commonly used for descending and thoracoabdominal aortic (DTAA) operations, mitigating the deleterious effects of cross-clamping. We present our initial experience performing DTAA replacement under LA-FA (left-to-left) cardiopulmonary bypass (CPB) with an oxygenator. DTAA replacement under LA-FA bypass with an oxygenator was performed in 14 consecutive patients (CPB group). The pulmonary vein and femoral artery (or distal aorta) were cannulated and the full CPB machine were used, including oxygenator, roller pump, pump suckers, and kinetically enhanced drainage. The CPB group was compared with 50 consecutive patients who underwent DTAA replacement utilizing traditional LA-FA bypass without an oxygenator (LA-FA group). -

The Mayo Gibbon Heart-Lung Bypass Machine in the Marc
Mayo Clinic Proceedings Legacy December 2014 Pioneers in Cardiac Surgery: The Mayo Gibbon Heart-Lung Bypass Machine This preliminary report shared the results from animal studies, but by May of that same year, Mayo Clinic had initiated use of the apparatus for human subjects and reported on the outcome in 8 cases.2 These patients had the following conditions: (1) ventricular septal defect, (2) Tetralogy of Fallot, or (3) persistent common atrioventricular canal. Four of the patients survived the surgery, one died shortly after the procedure, two died 3 hours postoperatively, and one died 6 days postoperatively.2 Mayo Clinic continued to study and develop successful surgical treatments in cardiology throughout the ensuing years. More than 60 years have passed since these first attempts, and success in cardiac surgery is directly related to these courageous research pioneers and patients. 1,2,3 This historical line of research in In the March 13, 1955, issue of The Proceedings cardiology also represents another successful of the Staff Meetings of the Mayo Clinic, Dr collaboration between the field of medicine John W. Kirklin and colleagues shared a and industry. International Business Machines preliminary report of their research from the (IBM) aided in the design and development of previous 3 years which tackled the challenges the Mayo Gibbon Heart-Lung Bypass of cardiac surgery. While other types of Machine.1,2,3 surgeries have their own impediments, cardiac surgery is dominated by the need to keep a References patient alive during the procedure while 1. Jones RE, Donald DE, Swan HJ, Harshbarger HG, maintaining the function of the circulatory Kirklin JW, Wood EH. -

Principles of Oxygenator Function: Gas Exchange, Heat Transfer, and Operation
Thoracic Key Fastest Thoracic Insight Engine Home Log In Register Categories » More References » About Gold Membership Contact Search... Principles of Oxygenator Function: Gas Exchange, Heat Transfer, and Operation Principles of Oxygenator Function: Gas Exchange, Heat Transfer, and Operation Michael H. Hines INTRODUCTION Since the early 1950s when the development of a heart-lung machine first began, there has been a tremendous evolution of devices and machinery for cardiac support (1,2). However, despite the diversity in designs through the years, they all contain three essential components: a mechanism to circulate the blood, a method of gas exchange for oxygen and carbon dioxide, and some mechanism for temperature control. Chapter 2 has covered the first important component, and we will now focus on the two subsequent elements: gas exchange and heat transfer. And while it is referred to as the “oxygenator,” we must recognize that it is responsible for the movement of both oxygen in, as well as carbon dioxide out. The discussion will start with a basic review of the principles of physics, and then we will apply those principles to the devices used specifically in extracorporeal support, including cardiopulmonary bypass (CPB) and extracorporeal membrane oxygenation (ECMO). You may notice as you go through this chapter that there is a scarcity of trade and manufacturer names. The author has intentionally avoided using these. The intent was primarily to focus on the physiology, physics, and chemistry of the oxygenator and heat exchanger, but also to emphasize the fact that there are a large number of manufacturers producing many products that have all been shown to function extremely well. -
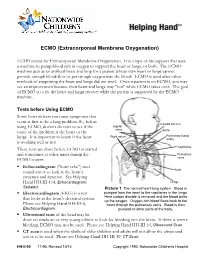
ECMO (Extracorporeal Membrane Oxygenation)
ECMO (Extracorporeal Membrane Oxygenation) ECMO stands for Extracorporeal Membrane Oxygenation. It is a type of life support that uses a machine to pump blood rich in oxygen to support the heart or lungs, or both. The ECMO machine acts as an artificial heart and lung for a patient whose own heart or lungs cannot provide enough blood flow or get enough oxygen into the blood. ECMO is used after other methods of supporting the heart and lungs did not work. Once a patient is on ECMO, you may see an improvement because their heart and lungs may "rest" while ECMO takes over. The goal of ECMO is to let the heart and lungs recover while the patient is supported by the ECMO machine. Tests before Using ECMO Some heart defects can cause symptoms that seem at first to be a lung problem. So, before Carotid arteries using ECMO, doctors do tests to see if the Jugular veins cause of the problem is the heart or the Aorta Right atrium Pulmonary (lung) lungs. It is important to know if the heart artery is working well or not. Capillaries These tests are done before ECMO is started and sometimes at other times during the Pulmonary ECMO course: veins . Echocardiogram ("heart echo") uses Heart sound waves to look at the heart’s structure and function. See Helping Hand HH-III-114, Echocardiogram: Lungs Sedated. Picture 1 The normal heart-lung system. Blood is . Electrocardiogram (EKG) is a test pumped from the heart to the capillaries in the lungs. that looks at the heart's electrical system. -

Cardiopulmonary Bypass Heart-Lung Machine Console
Food and Drug Administration, HHS § 870.4290 § 870.4220 Cardiopulmonary bypass or pieces of foreign material flowing in heart-lung machine console. the bloodstream which will obstruct (a) Identification. A cardiopulmonary circulation by blocking a vessel) out of bypass heart-lung machine console is a the blood. It is used in the arterial re- device that consists of a control panel turn line. and the electrical power and control (b) Classification. Class II (special circuitry for a heart-lung machine. The controls). The special control for this console is designed to interface with device is the FDA guidance document the basic units used in a gas exchange entitled ‘‘Guidance for system, including the pumps, Cardiopulmonary Bypass Arterial Line oxygenator, and heat exchanger. Blood Filter 510(k) Submissions.’’ (b) Classification. Class II (perform- ance standards). [45 FR 7907–7971, Feb. 5, 1980, as amended at 52 FR 17737, May 11, 1987; 66 FR 18542, Apr. 10, § 870.4230 Cardiopulmonary bypass 2001] defoamer. (a) Identification. A cardiopulmonary § 870.4270 Cardiopulmonary bypass bypass defoamer is a device used in cardiotomy suction line blood filter. conjunction with an oxygenator during (a) Identification. A cardiopulmonary cardiopulmonary bypass surgery to re- bypass cardiotomy suction line blood move gas bubbles from the blood. filter is a device used as part of a gas (b) Classification. Class II (special exchange (oxygenator) system to filter controls). The special control for this nonbiologic particles and emboli (a device is the FDA guidance document blood clot or a piece of foreign mate- entitled ‘‘Guidance for Extracorporeal rial flowing in the bloodstream which Blood Circuit Defoamer 510(k) Submis- will obstruct circulation by blocking a sions.’’ vessel) out of the blood. -

An Improved Technique for Isolated Perfusion of Rat Livers and an Evaluation of Perfusates HANS J
JOUHNAL OF SURGICAL RESEARCH 53, 158-165 (1992) An Improved Technique for Isolated Perfusion of Rat Livers and an Evaluation of Perfusates HANS J. MISCHINGER, M.D.,t THOMAS R. WALSH, M.D.,2 TAO Lm, M.D., PRAKASH N. RAO, PH.D., RANDY RUBIN, B.S., KENJIRO NAKAMURA, M.D., SATORU TODO, M.D., AND THOMAS E. STARZL, M.D., PH.D.3 Department of SurRery, University of PittsburRh, School of Medicine. Pittsburgh. Pennsylvania 15213 Submitted for publication April 27, 1990 ity of investigators have used variations of the original We have modified the apparatus for isolated rat liver perfusion arrangement introduced by Brower and Miller perfusion (IPRL) in order to be able to perform two [1-6]. At our laboratories this organ perfusion technique perfusions simultaneously. In addition, we studied the is performed to assess the viability of preserved livers quality and stability of livers by comparison of five dif prior to transplantation. ferent perfusates: Blood (Group A), Original Krebs To enable us to perfuse livers more efficiently, we con Henseleit buffer (Group B), Krebs buffer with glucose structed an IPRL apparatus designed to perfuse two (Group C) or bovine serum albumin (BSA) added, livers simultaneously. This perfusion arrangement al (Group D). In a last group (E) albumin, glucose, and lows more studies to be performed in less time and simul taurocholic acid were added to Krebs. After 180 min of taneous comparisons of different treatment regimens. perfusion, livers perfused with solutions including 2% This manuscript concerns the practical aspects of this albumin (Group D, E) had a significantly higher release of hepatocellular and endothelial cell (purine nucleo model as well as a study which compares stability of side phosphorylase) enzymes and lower bile production livers during perfusions with homologous blood, and as compared to Groups A, B, and C (P < 0.0001). -

Physiological Basis of Extracorporeal Membrane Oxygenation and Extracorporeal Carbon Dioxide Removal in Respiratory Failure
membranes Review Physiological Basis of Extracorporeal Membrane Oxygenation and Extracorporeal Carbon Dioxide Removal in Respiratory Failure Barbara Ficial 1, Francesco Vasques 1, Joe Zhang 1, Stephen Whebell 1 , Michael Slattery 1, Tomas Lamas 2 , Kathleen Daly 1, Nicola Agnew 1 and Luigi Camporota 1,3,* 1 Department of Adult Critical Care, Guy’s and St. Thomas’ NHS Foundation Trust, King’s Health Partners, London SE1 7EH, UK; barbara.fi[email protected] (B.F.); [email protected] (F.V.); [email protected] (J.Z.); [email protected] (S.W.); [email protected] (M.S.); [email protected] (K.D.); [email protected] (N.A.) 2 Department of Critical Care, Unidade de Cuidados Intensivos Polivalente, Egas Moniz Hospital, Rua da Junqueira 126, 1300-019 Lisbon, Portugal; [email protected] 3 Division of Centre of Human Applied Physiological Sciences, King’s College London, London SE1 7EH, UK * Correspondence: [email protected] Abstract: Extracorporeal life support (ECLS) for severe respiratory failure has seen an exponential growth in recent years. Extracorporeal membrane oxygenation (ECMO) and extracorporeal CO2 removal (ECCO2R) represent two modalities that can provide full or partial support of the native lung function, when mechanical ventilation is either unable to achieve sufficient gas exchange to meet metabolic demands, or when its intensity is considered injurious. While the use of ECMO has Citation: Ficial, B.; Vasques, F.; defined indications in clinical practice, ECCO2R remains a promising technique, whose safety and Zhang, J.; Whebell, S.; Slattery, M.; efficacy are still being investigated. Understanding the physiological principles of gas exchange Lamas, T.; Daly, K.; Agnew, N.; during respiratory ECLS and the interactions with native gas exchange and haemodynamics are Camporota, L. -

An Introduction to Extracorporal Circulation During Open Heart Surgery
An introduction to extracorporal circulation during open heart surgery 1. Introduction During open heart surgery, the surgical intervention is typically performed on a non-beating heart, and hence requires establishment of extracorporal circulation (ECC). During ECC, a heart-lung-machine temporarily replaces the functionality of the heart and lungs. This technique is therefore also called cardio pulmonary bypass (CPB). During closed heart surgery, the incision is performed without use of a heart-lung-machine. Coronary bypass operations can therefore in a number of cases be performed on a beating heart without use of ECC. The method is called OPCAP (Off Pump Coronary Artery Bypass). 2. The Primary Extra Corporal circuit During ECC the blood from the right side of the heart is drained through a venous catheter to a venous reservoir. Following this, the blood is pumped to an artificial lung – the oxygenator – where oxygen is added and carbondioxide is washed out. Finally the blood is pumped back into the systemic circulation by an arterial canulla in aorta ascendens. The oxygenator has a built-in heat-exchange system, which gives the blood the desired temperature. The individual components in the primary extracorporal circuit are described below from veneous catheter to arterial canulla. Figure 1. The primary extra corporal circuit. The right side of the heart is drained through a venous catheter to a venous reservoir. Following this, the blood is pumped to an artificial lung – the oxygenator – where oxygen is added and carbon dioxide is washed out. The oxygenated blood is pumped back into the systemic circulation through an arterial filter and by an arterial canulla in aorta ascendens. -

Guidance for Cardiopulmonary Bypass Oxygenators 510(K) Submissions; Final Guidance for Industry and FDA Staff
Guidance for Cardiopulmonary Bypass Oxygenators 510(k) Submissions; Final Guidance for Industry and FDA Staff Document issued on: November 13, 2000 This document supersedes Guidance for Cardiopulmonary Bypass Oxygenators 510(k) Submissions; Final January 17, 2000 U.S. Department of Health and Human Services Food and Drug Administration Center for Devices and Radiological Health Circulatory Support and Prosthetic Devices Branch Division of Cardiovascular and Respiratory Devices Office of Device Evaluation Preface Public Comment: Comments and suggestions may be submitted at any time for Agency consideration to Dockets Management Branch, Division of Management Systems and Policy, Office of Human Resources and Management Services, Food and Drug Administration, 5630 Fishers Lane, Room 1061, (HFA-305), Rockville, MD, 20852. When submitting comments, please refer to the exact title of this guidance document. Comments may not be acted upon by the Agency until the document is next revised or updated. For questions regarding the use or interpretation of this guidance contact Catherine Wentz at (240) 276-4141 or by e-mail at [email protected]. Additional Copies: Additional copies are available from the Internet at: http://www.fda.gov/cdrh/ode/guidance/1361.pdf or CDRH Facts-on-Demand. In order to receive this document via your fax machine, call the CDRH Facts-On-Demand system at 800-899-0381 or 301-827-0111 from a touch-tone telephone. Press 1 to enter the system. At the second voice prompt, press 1 to order a document. Enter the document number 1361 followed by the pound sign (#). Follow the remaining voice prompts to complete your request. -

A History of Innovation: Cardiac Surgery in Minnesota
RETROSPECTIVE A LOOK BACK AT MEDICINE A History of Innovation CARDIAC SURGERY IN MINNESOTA COURTESYPHOTO OF MINNESOTA UNIVERSITY ABOVE: C. Walton BY JOHNATHON M. AHO, MD, MATTHEW S. SCHAFF, MD, CORNELIUS A. THIELS, DO, MBA, ROBERT A. DARLING, MD, Lillehei and team MARK N. PRICE KOERNER AND HARTZELL V. SCHAFF, MD performing a controlled cross-circulation For centuries, the heart was believed to be an inoperable organ. Through the development of new technologies operation in the mid-1950s. and techniques, the initial difficulties inherent with operating on a moving organ began to fade. But as surgeons in the last century pushed the boundaries of cardiac repair, new problems arose. To solve them, they enlisted the help of physiologists, residents and engineers. By taking a multidisciplinary approach, sharing information and ideas, and working collaboratively, University of Minnesota and Mayo Clinic investigators found themselves at the forefront of cardiac surgery. This article reviews Minnesota’s contributions to the field. “There, for a shining moment, the THE EARLY YEARS ment of shrapnel.6 The early successes of only institutions where one could As surgery involving other organs ad- Harken and other combat surgeons as well as vanced, Aristotle’s conviction that the the development of extracardiac procedures go for open heart surgery were heart was inoperable prevailed and most such as closure of the patent ductus arterio- 90 miles apart, at the Mayo Clinic surgeons viewed operating on the heart as sus in 1939, repair of an aortic coarctation and the University of Minnesota.” taboo.3 That thinking was challenged to in 1945 and development of the Blalock- some extent in the early 19th century with Taussig shunt for treating cyanotic heart — Norman Shumway, MD the development of extracardiac procedures disease in the 1940s convinced surgeons that for treating penetrating thoracic injuries. -

John Heysham Gibbon and the 60Th Anniversary of the First Successful Heart-Lung Machine: Brief Notes About the Development of Cardiac Surgery in Europe and Slovakia
DOI: 10.4149/BLL_2013_051 Bratisl Lek Listy 2013; 114 (5) 247–250 THE DESTINY OF PROMINENT PHYSICIANS John Heysham Gibbon and the 60th Anniversary of the First Successful Heart-Lung Machine: Brief Notes about the Development of Cardiac Surgery in Europe and Slovakia Silvay G, Castillo JG Department of Anesthesiology, The Mount Sinai School of Medicine, New York, USA. [email protected] Abstract: The development of the heart-lung machine and its fi rst successful clinical application in 1953 was the culmination of Dr. Gibbon’s lifetime research project. Despite many technical obstacles, fi nancial prob- lems, and discouragement from colleagues, his goal was achieved after twenty tedious years of tireless work. Posteriorly, his academic contribution established him as a leader and pioneer in the fi eld of cardiac surgery. Parallel to his achievement and Dr. Kirklin’s surgical experience, several authors around the world attempted open-heart surgery with the heart-lung machine, particularly in Europe. In Eastern Europe and particularly in the former Czechoslovakia, the lack of access to foreign medical literature forced a group of emerging young physicians from the Second Department of Surgery at Comenius University to furtively collect data on the topic. After building the Simkovic-Bolf heart-lung machine, the fi rst successful open-heart surgery with the new device was performed only 5 years after Dr. Gibbons’ experience (Tab. 1, Fig. 4, Ref. 22). Full Text in PDF www.elis.sk. Key words: heart-lung machine, cardiac surgery, pioneer in the fi eld of cardiac surgery. Sixty years ago, at the Jefferson Hospital in Philadelphia, doc- tor John H.