Advanced Lost Foam Casting Technology Phase Iv
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Compositions for Ceramic Cores Used in Investment Casting
(19) TZZ¥_Z_T (11) EP 3 170 577 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 24.05.2017 Bulletin 2017/21 B22C 9/10 (2006.01) (21) Application number: 16199374.6 (22) Date of filing: 17.11.2016 (84) Designated Contracting States: • LEMAN, John Thomas AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Niskayuna, NY 12309 (US) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • KU, Anthony Yu-Chung PL PT RO RS SE SI SK SM TR Niskayuna, NY 12309 (US) Designated Extension States: • LI, Tao BA ME Evendale, OH 45215 (US) Designated Validation States: • POLLINGER, John Patrick MA MD Niskayuna, NY 12309 (US) (30) Priority: 19.11.2015 US 201514945602 (74) Representative: Pöpper, Evamaria General Electric Technology GmbH (71) Applicant: General Electric Company GE Corporate Intellectual Property Schenectady, NY 12345 (US) Brown Boveri Strasse 7 5400 Baden (CH) (72) Inventors: • YANG, Xi Alpha, OH 45301 (US) (54) COMPOSITIONS FOR CERAMIC CORES USED IN INVESTMENT CASTING (57) The present disclosure generally relates to a ce- body, but is largely unavailable for reaction with metal ramic core comprising predominantly mullite, which is alloys used in investment casting. Methods of making derived from a precursor comprising alumina particles cast metal articles are also disclosed. and siloxane binders. Free s ilica is present in the ceramic EP 3 170 577 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 170 577 A1 Description TECHNICAL FIELD 5 [0001] The present disclosure generally relates to compositions for investment casting cores and methods for making them. -
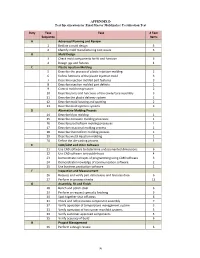
APPENDIX D Test Specification for Final Master Moldmaker Certification Test
APPENDIX D Test Specification for Final Master Moldmaker Certification Test Duty Task Task # Test Sequence Items A Advanced Planning and Review 1 Redline a mold design 5 2 Identify mold manufacturing cost issues 3 B Mold Design 3 Check mold components for fit and function 6 4 Design jigs and fixtures 4 C Plastic Injection Molding 5 Describe the process of plastic injection molding 2 6 Define functions of the plastic injection mold 3 7 Describe injection molded part features 3 8 Describe injection molded part defects 2 9 Control mold temperature 2 10 Describe parts and functions of the cavity/core assembly 3 11 Describe the plastic delivery system 2 12 Describe mold locating and spotting 2 13 Describe mold ejection systems 3 D Alternative Molding Process 14 Describe blow molding 1 15 Describe extrusion molding processes 1 16 Describe plastic/foam molding processes 1 17 Describe rotational molding process 1 18 Describe thermoform molding process 1 19 Describe metal injection molding 1 20 Define the die‐casting process 1 E CAD/CAM and other Software 21 Use CAD software to determine undocumented dimensions 6 22 Use CAD software to troubleshoot 4 23 Demonstrate concepts of programming using CAM software 3 24 Demonstrate knowledge of communication software 2 25 Use business production software 1 F Inspection and Measurement 26 Request and verify part dimensions and features data 6 27 Perform in‐process checks 13 G Assembly, Fit and Finish 28 Bench and polish steel 6 29 Perform or request specialty finishing 3 30 Spot together shut off areas -

Boilermaking Manual. INSTITUTION British Columbia Dept
DOCUMENT RESUME ED 246 301 CE 039 364 TITLE Boilermaking Manual. INSTITUTION British Columbia Dept. of Education, Victoria. REPORT NO ISBN-0-7718-8254-8. PUB DATE [82] NOTE 381p.; Developed in cooperation with the 1pprenticeship Training Programs Branch, Ministry of Labour. Photographs may not reproduce well. AVAILABLE FROMPublication Services Branch, Ministry of Education, 878 Viewfield Road, Victoria, BC V9A 4V1 ($10.00). PUB TYPE Guides Classroom Use - Materials (For Learner) (OW EARS PRICE MFOI Plus Postage. PC Not Available from EARS. DESCRIPTORS Apprenticeships; Blue Collar Occupations; Blueprints; *Construction (Process); Construction Materials; Drafting; Foreign Countries; Hand Tools; Industrial Personnel; *Industrial Training; Inplant Programs; Machine Tools; Mathematical Applications; *Mechanical Skills; Metal Industry; Metals; Metal Working; *On the Job Training; Postsecondary Education; Power Technology; Quality Control; Safety; *Sheet Metal Work; Skilled Occupations; Skilled Workers; Trade and Industrial Education; Trainees; Welding IDENTIFIERS *Boilermakers; *Boilers; British Columbia ABSTRACT This manual is intended (I) to provide an information resource to supplement the formal training program for boilermaker apprentices; (2) to assist the journeyworker to build on present knowledge to increase expertise and qualify for formal accreditation in the boilermaking trade; and (3) to serve as an on-the-job reference with sound, up-to-date guidelines for all aspects of the trade. The manual is organized into 13 chapters that cover the following topics: safety; boilermaker tools; mathematics; material, blueprint reading and sketching; layout; boilershop fabrication; rigging and erection; welding; quality control and inspection; boilers; dust collection systems; tanks and stacks; and hydro-electric power development. Each chapter contains an introduction and information about the topic, illustrated with charts, line drawings, and photographs. -

Metal Casting Terms and Definitions
Metal Casting Terms and Definitions Table of Contents A .................................................................................................................................................................... 2 B .................................................................................................................................................................... 2 C .................................................................................................................................................................... 2 D .................................................................................................................................................................... 4 E .................................................................................................................................................................... 5 F ..................................................................................................................................................................... 5 G .................................................................................................................................................................... 5 H .................................................................................................................................................................... 6 I .................................................................................................................................................................... -

Ironworks and Iron Monuments Forges Et
IRONWORKS AND IRON MONUMENTS FORGES ET MONUMENTS EN FER I( ICCROM i ~ IRONWORKS AND IRON MONUMENTS study, conservation and adaptive use etude, conservation et reutilisation de FORGES ET MONUMENTS EN FER Symposium lronbridge, 23-25 • X •1984 ICCROM rome 1985 Editing: Cynthia Rockwell 'Monica Garcia Layout: Azar Soheil Jokilehto Organization and coordination: Giorgio Torraca Daniela Ferragni Jef Malliet © ICCROM 1985 Via di San Michele 13 00153 Rome RM, Italy Printed in Italy Sintesi Informazione S.r.l. CONTENTS page Introduction CROSSLEY David W. The conservation of monuments connected with the iron and steel industry in the Sheffield region. 1 PETRIE Angus J. The No.1 Smithery, Chatham Dockyard, 1805-1984 : 'Let your eye be your guide and your money the last thing you part with'. 15 BJORKENSTAM Nils The Swedish iron industry and its industrial heritage. 37 MAGNUSSON Gert The medieval blast furnace at Lapphyttan. 51 NISSER Marie Documentation and preservation of Swedish historic ironworks. 67 HAMON Francoise Les monuments historiques et la politique de protection des anciennes forges. 89 BELHOSTE Jean Francois L'inventaire des forges francaises et ses applications. 95 LECHERBONNIER Yannick Les forges de Basse Normandie : Conservation et reutilisation. A propos de deux exemples. 111 RIGNAULT Bernard Forges et hauts fourneaux en Bourgogne du Nord : un patrimoine au service de l'identite regionale. 123 LAMY Yvon Approche ethnologique et technologique d'un site siderurgique : La forge de Savignac-Ledrier (Dordogne). 149 BALL Norman R. A Canadian perspective on archives and industrial archaeology. 169 DE VRIES Dirk J. Iron making in the Netherlands. 177 iii page FERRAGNI Daniela, MALLIET Jef, TORRACA Giorgio The blast furnaces of Capalbio and Canino in the Italian Maremma. -

Lost Foam Casting
Emerging Technology and Successful Partnerships: Lost Foam Casting Harvey Wong, U.S. Department ofEnergy Kenneth M Green, BCS, Incorporated ABSTRACT In 1989, a consortium offoundries, suppliers, and academiajoined the U~So Department ofEnergy (DOE) .. in a research program to improve c'ontrol over the lost foam casting process thereby reducing defects and producing high quality precision castings. Lost foam casting is a process that offers many energy and environmental advantages and enables the production of complex parts. The government side,ofthis partnership is led by the u.s. DepartmentofEnergy (DOE), Office ofEnergy Efficiency and Renewable Energy, Office ofIndustrial Technologies (OIT), Metal Casting Industry ofthe Future. Research is conducted through the Lost Foam Technology Center at the University of Alabama em Birmingham. The Consortium has been credited as the driving force behind technical improvements in the lost foam casting process.. Ithas improved knowledge andcontrol inall stages oflostfoam casting.. The LostFoam Casting research was recently profiled by the International Energy Agency, Centre for the Analysis and DisselninationofDemonstrated Energy Technologies (CADDET). Lostfoam casting isjustone of many metal casting techniques. The Metal Casting Industry of the Future partnership is conducting research to improve productivity and energy efficiency innearly all areas ofcasting.. Usingthe ongoing Metal Casting Industry ofthe Future lostfoam research as anexample, paper discusses the be ts ofindustry-government consortia advancing R&D for critical U"S .. industries such as metal casting.. It describes how such partnerships can be pivotal in helping to move emerging technologies from concept to implementation~ Introduction metalcasting industry is vital to the U.S. 1\./lIt::lA'tt:1lmr"':'llC""t'llnn ~n~u"ul::li>CI the to complex parts to meet a variety engine blocks to plumbing fixtures. -

Section 02 Tooling for Die Casting
Tooling for Die Casting SECTION Section Contents NADCA No. Format Page Frequently Asked Questions (FAQ) 2-2 2 1 Introduction 2-3 2 Types of Die Casting Dies 2-4 2.1 Prototyping 2-4 2 2.2 Rapid Tooling Dies 2-4 2.3 Production Dies 2-5 2.4 Unit Dies 2-6 2.5 Trim Dies 2-6 3 Casting Features and Die Considerations 2-7 3.1 Core Slide Requirements 2-8 3.2 Parting Line: Cover & Ejector Die Halves 2-8 3.3 Ejector Pins 2-9 3.4 Cast-in Inserts 2-9 4 Die Materials 2-10 4.1 Die and Cavity Materials 2-10 4.2 Die Cavity Insert Materials 2-10 4.3 Die Steel Heat Treatment 2-11 5 Controlling Die Performance 2 -11 5.1 Porosity Control: Gating, Venting, Vacuum 2-11 5.2 Thermal Balancing 2-12 5.3 Oil Heating Lines 2-12 5.4 Alternate Surface Textures 2-12 5.5 Extended Die Life 2-12 6 Secondary Machining Preplanning 2-14 7 Gaging Considerations 2-14 8 Inherited Tooling 2-15 9 Engineering Consultation 2-15 10 Database Guidelines 2-16 11 New Die/Inherited Die Specifications 2-16 12 Die Life 2-16 13 Checklist for Die Casting Die Specifications T-2-1-12 Checklist 2-17 14 Guidelines to Increase Die Life T-2-2-12 Guideline 2-19 NADCA Product Specification Standards for Die Castings / 2015 2-1 Tooling for Die Casting A-PARTING LINE Surface where two die halves Frequently Asked Questions (FAQ) come together. -

A Study of Mixed Manufacturing Methods in Sand Casting Using 3D Sand Printing and FDM Pattern-Making Based on Cost and Time by R
A Study of Mixed Manufacturing Methods in Sand Casting Using 3D Sand Printing and FDM Pattern-making Based on Cost and Time by Ram A. Gullapalli Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Industrial and Systems Engineering Program Youngstown State University Dec, 2016 A Study of Mixed Manufacturing Methods in Sand Casting Using 3D Sand Printing and FDM Pattern-Making Based on Cost and Time By Ram A. Gullapalli I hereby release this thesis to the public. I understand that this thesis will be made available from the OhioLINK ETD Center and the Maag Library Circulation Desk for public access. I also authorize the University or other individuals to make copies of this thesis as needed for scholarly research. Signature: Ram A. Gullapalli, Student Date Approvals: Dr. Brett Conner, Thesis Advisor Date Dr. Darrell Wallace, Committee Member Date Dr. Eric MacDonald, Committee Member Date Dr. Sal Sanders, Dean of Graduate Studies Date Abstract Sand casting has long been known to be an effective manufacturing method for metal casting and especially for parts of large dimensions and low production volume. But, for increasing complexity, the conventional sand casting process does have its limitations; one of them mainly being the high cost of tooling to create molds and cores. With the advent of additive manufacturing (AM), these limitations can be overcome by the use of a 3D sand printer which offers the unique advantage of geometric freedom. Previous research shows the cost benefits of 3D sand printing molds and cores when compared to traditional mold and core making methods. -

Instructions for the Preparation Of
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Directory of Open Access Journals ISSN (1897-3310) ARCHIVES Volume 11 Special Issue of 1/2011 FOUNDRY ENGINEERING 5 – 10 Published quarterly as the organ of the Foundry Commission of the Polish Academy of Sciences 1/1 Hydrogen analysis and effect of filtration on final quality of castings from aluminium alloy AlSi7Mg0,3 M. Brůna*, A. Sládek Department of Technological Engineering, Žilina University from Žilina , Univerzitná 1825/1 010 26 Žilina, Slovakia *Corresponding author. E-mail address: [email protected] Received 21.02.2011; accepted in revised form 25.02.2011 Abstract The usage of aluminium and its alloys have increased in many applications and industries over the decades. The automotive industry is the largest market for aluminium castings and cast products. Aluminium is widely used in other applications such as aerospace, marine engines and structures. Parts of small appliances, hand tools and other machinery also use thousands of different aluminium castings. The applications grow as industry seeks new ways to save weight and improve performance and recycling of metals has become an essential part of a sustainable industrial society. The process of recycling has therefore grown to be of great importance, also another aspect has become of critical importance: the achievement of quality and reliability of the products and so is very important to understand the mechanisms of the formation of defects in aluminium melts, and also to have a reliable and simple means of detection. Keywords: Castings defects; Quality management; Aluminium; Filtration; Remelting quality from plant to plant, and, unfortunately, quality can also 1. -

3. Pattern, Mold and Core Design
3 . Pat t er n, Mold and Cor e D es ign The most important decision in pattern and mold design is about the parting line. It affects and is affected by part orientation, design of pattern and cores, number of cavities in the mold, location of feeders, and channels for gating, cooling and venting. In this chapter, we will first develop a scientific definition of parting line, followed by the design of parting line, pattern, mold cavities and cores. 3.1 Parting L ine Des ign The parting or separation between two or more segments of a mold is necessary to create the mold cavity (as in sand casting) and also to remove the manufactured part from the mold (as in diecasting). For any given casting geometry, a number of parting alternatives may exist; visualizing and selecting the best alternative is a non-trivial task even for simple shapes. Variations in customer requirements, quality specifications, manufacturing facilities and economical considerations may lead to different parting solutions for the same shape. For intricate parts, there is a high possibility of overlooking feasible alternatives and difficulty in assuring that the selected alternative is the indeed the best one. To evolve a scientific approach to parting line design and analysis, unambiguous definitions of parting and related features are required, valid for all types of tooling being considered. The following definitions are proposed. Mold segment is a distinct body, at least one face of which is in contact with the casting. Draw direction of a mold segment is the direction along which it is withdrawn from the adjacent mold segment. -

Modification Mechanism of Spinel Inclusions in Medium Manganese
metals Article Modification Mechanism of Spinel Inclusions in Medium Manganese Steel with Rare Earth Treatment Zhe Yu 1,2 and Chengjun Liu 1,2,* 1 Key Laboratory for Ecological Metallurgy of Multimetallic Ores (Ministry of Education), Shenyang 110819, China 2 School of Metallurgy, Northeastern University, Shenyang 110819, China * Correspondence: [email protected]; Tel.: +86-138-9885-0333 Received: 7 July 2019; Accepted: 19 July 2019; Published: 21 July 2019 Abstract: In aluminum deoxidized medium manganese steel, spinel inclusions are easily to form during refining, and such inclusions will deteriorate the toughness of the medium manganese steel. Rare earth inclusions have a smaller hardness, and their thermal expansion coefficients are similar to that of steel. They can avoid large stress concentrations around inclusions during the heat treatment of steel, which is beneficial for improving the toughness of steel. Therefore, rare earth Ce is usually used to modify spinel inclusions in steel. In order to clarify the modification mechanism of spinel inclusions in medium manganese steel with Ce treatment, high-temperature simulation experiments were carried out. Samples were taken step by step during the experimental steel smelting process, and the inclusions in the samples were analyzed by SEM-EDS. Finally, the experimental results were discussed and analyzed in combination with thermodynamic calculations. The results show that after Ce treatment, the amount of inclusions decrease, the inclusion size is basically less than 5 µm, and the spinel inclusions are transformed into rare earth inclusions. After Ce addition, Mn and Mg in the spinel inclusions are first replaced by Ce, and the spinel structure is destroyed to form CeAlO3. -

Status Quo and Development Trend of Lost Foam Casting Technology
Special Report Celebrating the th Anniversray 2004-2014 CHINA FOUNDRY Vol.11 No.4 July 2014 10 Status quo and development trend of lost foam casting technology *Fan Zitian1, Jiang Wenming1,2, Liu Fuchu1, and Xiao Botao1 1. State Key Laboratory of Material Processing and Die & Mould Technology, Huazhong University of Science and Technology, Wuhan 430074, China; 2. School of Mechanical & Electrical Engineering, Wuhan Institute of Technology, Wuhan 430073, China Abstract: Lost foam casting (LFC) technology has been widely applied to cast iron and cast steel. However, the development of LFC for Al and Mg alloys was relatively slower than that for cast iron and cast steel. The application of LFC to Al and Mg alloys needs more effort, especially in China. In this paper, the development history of LFC is reviewed, and the application situations of LFC to Al and Mg alloys are mainly discussed. Meanwhile, the key problems of LFC for Al and Mg alloys are also pointed out. Finally, the prospects for LFC technology are discussed, and some special new LFC technologies are introduced for casting Al and Mg alloys. In future, the development trends of green LFC technology mainly focus on the special new LFC methods, metal material, coating, heat treatment, new foam materials as well as purification technology of tail gas, etc. Key words: lost foam casting (LFC); technology actuality; development trend; aluminum alloy; magnesium alloy CLC numbers: TG249.5 *Fan Zitian Document code: A Born in 1962 Ph.D, Professor. Article ID: 1672-6421(2014)04-296-12 He has been mainly concentrating on education, research and application of casting materials; process theory and equipment.