Groundtruthing and Potential for Predicting Acid Deposition Impacts in Headwater Streams Using Bedrock Geology, GIS, Angling, and Stream Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stratigraphic Framework of Cambrian and Ordovician Rocks in The
Stratigraphic Framework of Cambrian and Ordovician Rocks in the Central Appalachian Basin from Medina County, Ohio, through Southwestern and South-Central Pennsylvania to Hampshire County, West Virginia U.S. GEOLOGICAL SURVEY BULLETIN 1839-K Chapter K Stratigraphic Framework of Cambrian and Ordovician Rocks in the Central Appalachian Basin from Medina County, Ohio, through Southwestern and South-Central Pennsylvania to Hampshire County, West Virginia By ROBERT T. RYDER, ANITA G. HARRIS, and JOHN E. REPETSKI Stratigraphic framework of the Cambrian and Ordovician sequence in part of the central Appalachian basin and the structure of underlying block-faulted basement rocks U.S. GEOLOGICAL SURVEY BULLETIN 1839 EVOLUTION OF SEDIMENTARY BASINS-APPALACHIAN BASIN U.S. DEPARTMENT OF THE INTERIOR MANUEL LUJAN, Jr., Secretary U.S. GEOLOGICAL SURVEY Dallas L. Peck, Director Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government UNITED STATES GOVERNMENT PRINTING OFFICE: 1992 For sale by Book and Open-File Report Sales U.S. Geological Survey Federal Center, Box 25286 Denver, CO 80225 Library of Congress Cataloging in Publication Data Ryder, Robert T. Stratigraphic framework of Cambrian and Ordovician rocks in the central Appalachian Basin from Medina County, Ohio, through southwestern and south-central Pennsylvania to Hampshire County, West Virginia / by Robert T. Ryder, Anita C. Harris, and John E. Repetski. p. cm. (Evolution of sedimentary basins Appalachian Basin ; ch. K) (U.S. Geological Survey bulletin ; 1839-K) Includes bibliographical references. Supt. of Docs, no.: I 19.3:1839-K 1. Geology, Stratigraphic Cambrian. -

Structural Geology of the Transylvania Fault Zone in Bedford County, Pennsylvania
University of Kentucky UKnowledge University of Kentucky Master's Theses Graduate School 2009 STRUCTURAL GEOLOGY OF THE TRANSYLVANIA FAULT ZONE IN BEDFORD COUNTY, PENNSYLVANIA Elizabeth Lauren Dodson University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Dodson, Elizabeth Lauren, "STRUCTURAL GEOLOGY OF THE TRANSYLVANIA FAULT ZONE IN BEDFORD COUNTY, PENNSYLVANIA" (2009). University of Kentucky Master's Theses. 621. https://uknowledge.uky.edu/gradschool_theses/621 This Thesis is brought to you for free and open access by the Graduate School at UKnowledge. It has been accepted for inclusion in University of Kentucky Master's Theses by an authorized administrator of UKnowledge. For more information, please contact [email protected]. ABSTRACT OF THESIS STRUCTURAL GEOLOGY OF THE TRANSYLVANIA FAULT ZONE IN BEDFORD COUNTY, PENNSYLVANIA Transverse zones cross strike of thrust-belt structures as large-scale alignments of cross-strike structures. The Transylvania fault zone is a set of discontinuous right-lateral transverse faults striking at about 270º across Appalachian thrust-belt structures along 40º N latitude in Pennsylvania. Near Everett, Pennsylvania, the Breezewood fault terminates with the Ashcom thrust fault. The Everett Gap fault terminates westward with the Hartley thrust fault. Farther west, the Bedford fault extends westward to terminate against the Wills Mountain thrust fault. The rocks, deformed during the Alleghanian orogeny, are semi-independently deformed on opposite sides of the transverse fault, indicating fault movement during folding and thrusting. Palinspastic restorations of cross sections on either side of the fault zone are used to compare transverse fault displacement. -

Post-Martinsburg Ordovician Stratigraphy of Virginia and West Virginia
VIRGINIA DIVISION OF MINERAL RESOURCES PUBLICATION 57 POST-MARTINSBURG ORDOVICIAN STRATIGRAPHY OF VIRGINIA AND WEST VIRGINIA Richard J. Diecchio COMMONWEALTH OF VIRGINIA DEPARTMENT OF MINES, MINERALS AND ENERGY DIVISION OF MINERAL RESOURCES R<>bert C. Milici, Commissioner of Mineral Resources and State Geologist CHARLOTTESVI LLE, VIRG IN IA 1 985 VIRGINIA DIVISION OF MINERAL RESOURCES PUBLICATION 57 POST-MARTINSBURG ORDOVICIAN STRATIGRAPHY OF VIRGINIA AND WEST VIRGINIA Richard J. Diecchio COMMONWEALTH OF VIRGINIA DEPARTMENT OF MINES, MINERALS AND ENERGY DIVISION OF MINERAL RESOURCES Robert C. Milici, Commissioner of Mineral Resources and State Geologist CHARLOTTESVI LLE, VI RGI N IA 1 985 FRONT COVER: Two cycles within the Juniata Formation, Cumberland, Maryland. Jacob's staff is 5 feet long, graduated in feet. Bottom of staff marks base of lowermost sandstone bed (base of cycle). Basal sandstone is here channeled into the underlying mudstone. Top of Jacob's staff marks the middle Sholithos- bearing portion of cycle. Mudstone overlies the Skoli,thos facies and continues up to base of overlying sandstone bed, marking the base of the next cycle. VIRGINIA DIVISION OF MINERAL RESOURCES PUBLICATION 57 POST-MARTINSBURG ORDOVICIAN STRATIGRAPHY OF VIRGINIA AND WEST VIRGINIA Richard J. Diecchio COMMONWEALTH OF VIRGINIA DEPARTMENT OF MINES, MINERALS AND ENERGY DIVISION OF MINERAL RESOURCES Robert C. Milici, Commissioner of Mineral Resources and State Geologist CHARLOTTESVI LLE, VI RGI N IA 1985 DEPARTMENT OF MINES, MINERALS AND ENERGY Richmond, Virginia O. GENE DISHNER, Director COMMONWEALTH OF VIRGINIA DEPARTMENT OF PURCHASE AND SUPPLY RICHMOND 1985 Publication available from Virginia Division of Mineral Resources, Box 366?, Charlottesville, VA 22g0g. -

Geologic Resources Inventory Ancillary Map Information Document
U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Directorate Geologic Resources Division Allegheny Portage Railroad National Historic Site and Johnstown Flood National Memorial GRI Ancillary Map Information Document Produced to accompany the Geologic Resources Inventory (GRI) Digital Geologic-GIS Data for Allegheny Portage Railroad National Historic Site and Johnstown Flood National Memorial alpo_jofl_geology.pdf Version: 5/11/2020 I Allegheny Portage Railroad National Historic Site and Johnstown Flood National Memorial Geologic Resources Inventory Map Document for Allegheny Portage Railroad National Historic Site and Johnstown Flood National Memorial Table of Contents Geologic Resourc..e..s.. .I.n..v..e..n..t.o...r.y.. .M...a..p.. .D...o..c..u..m...e...n..t............................................................................ 1 About the NPS Ge..o..l.o..g..i.c... .R..e..s..o..u...r.c..e..s.. .I.n..v..e..n...t.o..r.y.. .P...r.o..g...r.a..m............................................................... 3 GRI Digital Maps a..n...d.. .S..o..u...r.c..e.. .M...a..p.. .C...i.t.a..t.i.o...n..s.................................................................................. 5 Index Map .................................................................................................................................................................... 6 Digital Bedrock Geologic-GIS Map of Allegheny Portage Railroad National Historic Site, John..s..t..o..w...n.. .F..l.o..o...d.. .N...a..t.i.o..n..a..l. .M...e..m...o...r.i.a..l. .a..n..d... .V..i.c..i.n..i.t.y.................................................. 7 Map Unit Lis.t................................................................................................................................................................... 7 Map Unit De.s..c..r.i.p..t.io..n..s...................................................................................................................................................... 8 PNcc - Cas.s..e..l.m...a..n. -

Hydrogeologic Setting and Conceptual Hydrologic Model of the Spring Creek Basin, Centre County, Pennsylvania, June 2005
Hydrogeologic Setting and Conceptual Hydrologic Model of the Spring Creek Basin, Centre County, Pennsylvania, June 2005 By John W. Fulton, Edward H. Koerkle, Steven D. McAuley, Scott A. Hoffman, and Linda F. Zarr In cooperation with the ClearWater Conservancy Scientific Investigations Report 2005-5091 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior Gale A. Norton, Secretary U.S. Geological Survey P. Patrick Leahy, Acting Director U.S. Geological Survey, Reston, Virginia: 2005 For sale by U.S. Geological Survey, Information Services Box 25286, Denver Federal Center Denver, CO 80225 For more information about the USGS and its products: Telephone: 1-888-ASK-USGS World Wide Web: http://www.usgs.gov/ Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to repro- duce any copyrighted materials contained within this report. Suggested citation: Fulton, J.W., Koerkle, E.H., McAuley, S.D., Hoffman, S.A., and Zarr, L.F., 2005, Hydrogeologic setting and conceptual hydrologic model of the Spring Creek Basin, Centre County, Pennsylvania, June 2005: U.S. Geological Survey Scientific Investigations Report 2005-5091, 83 p. iii Contents Abstract. 1 Introduction . 1 Purpose and Scope . 1 Description of Study Area . 1 Physical Characteristics of Spring Creek Basin . 3 Climate . 7 Precipitation. 7 Air Temperature . 12 Humidity. 12 Solar Radiation . 12 Evaporation . 12 Physiography . 13 Topography . 13 Land Use. 15 Hydrogeologic Setting of Spring Creek Basin . -

2007 Source Water Protection Report
State College Borough Water Authority State College, Pennsylvania Source Water Protection Report January 2007 State College Borough Water Authority 56002680 sjw-067 1-07 -i- STATE COLLEGE BOROUGH WATER AUTHORITY STATE COLLEGE, PENNSYLVANIA SOURCE WATER PROTECTION REPORT JANUARY 2007 PREPARED BY: DAVID A. YOXTHEIMER, P.G. APPROVED BY: ROBERT W. ANDERSON, C.P.G. 56002680 120 Radnor Road State College, Pennsylvania 16801 tel 814 231 2170· fax 814 231 2174 State College Borough Water Authority 56002680 sjw-067 1-07 -ii- Source Water Protection Report Table of Contents Page No. Section 1 Introduction .......................................................................................1 1.1 Purpose and Objectives ..................................................................................1 1.2 Background Information .................................................................................1 1.2.1 Hydrogeologic Setting..........................................................................2 1.2.2 Water System Description....................................................................3 1.2.3 Land Use and Zoning...........................................................................3 1.2.4 Previous Related Studies.....................................................................4 Section 2 Upper Slab Cabin Run Watershed Protection Study......................6 2.1 Literature and Data Review............................................................................6 2.2 Water Level Surveys ......................................................................................7 -
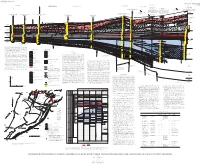
Stratigraphic Framework of Cambrian and Ordovician
U.S. DEPARTMENT OF THE INTERIOR U.S. GEOLOGICAL SURVEY MISCELLANEOUS INVESTIGATIONS SERIES MAP I-2200 WOOSTER ARCH UNNAMED HINGE ZONE SE NORTHEASTERN END OF ROME TROUGH (MAIN BRANCH) ROME TROUGH (MINOR BRANCH) ALLEGHENY STRUCTURAL B' OHIO-WEST VIRGINIA HINGE ZONE 9t 11t FRONT Shell Oil Company California Company 10t No. 1 Snyder No. 1 Shade Mountain NW Deep Rock Oil Corporation Juniata Co., Pa. 8 Lycoming Co., Pa. No. A-1 Walizer B Texaco Incorporated and Marathon Oil Company No. 1 (Restored about 40 mi (64 km) southeastward to prethrusting A-A' Clinton Co., Pa. position using section C-C' from Berg and others (1980); bed Pennsylvania State Forest Tract 285 Composite outcrop section above drill hole Thrust faults showing about from Rones (1969) and Faill and Wells (1977) Composite outcrop section above drill hole thicknesses corrected for 45˚ dip above 4540 ft; gamma-ray Clinton Co., Pa 8 mi (12.9 km) shortening log above 4540 ft has not been corrected for dip) (Restored about 10 mi (16 km) southeastward from Thompson (1963), Rones (1969), Faill (Berg and others, 1980) Tie with to prethrusting position using section from Faill and Wells (1977), and Cotter (1982) Thrust faults showing about and Wells (1977); dip approximately horizontal, (Restored about 19 mi (30.6 km) southeast- LOWER (R.T. Ryder, unpub. data) unpub. Ryder, (R.T. FEET 8.5 mi (13.7 km) shortening thus, bed thickness correction unnecessary) ward to prethrusting position using section SILURIAN 7 10,000 from Faill and Wells (1977); bed thicknesses 1 (Faill and Wells, 1977) 2 4 Consolidated Gas Supply Corporation No. -

Early Silurian Terrestrial Biotas of Virginia, Ohio, and Pennsylvania: an Investigation Into the Early Colonization of Land (284 Pp.)
LATE ORDOVICIAN – EARLY SILURIAN TERRESTRIAL BIOTAS OF VIRGINIA, OHIO, AND PENNSYLVANIA: AN INVESTIGATION INTO THE EARLY COLONIZATION OF LAND A dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Alexandru Mihail Florian Tomescu November 2004 © 2004 Alexandru Mihail Florian Tomescu All Rights Reserved This dissertation entitled LATE ORDOVICIAN – EARLY SILURIAN TERRESTRIAL BIOTAS OF VIRGINIA, OHIO, AND PENNSYLVANIA: AN INVESTIGATION INTO THE EARLY COLONIZATION OF LAND BY ALEXANDRU MIHAIL FLORIAN TOMESCU has been approved for the Department of Biological Sciences and the College of Arts and Sciences by Gar W. Rothwell Distinguished Professor of Environmental and Plant Biology Leslie A. Flemming Dean, College of Arts and Sciences TOMESCU, ALEXANDRU MIHAIL FLORIAN. Ph.D. November 2004. Biological Sciences Late Ordovician – Early Silurian terrestrial biotas of Virginia, Ohio, and Pennsylvania: an investigation into the early colonization of land (284 pp.) Director of Dissertation: Gar W. Rothwell An early phase in the colonization of land is documented by investigation of three fossil compression biotas from Passage Creek (Silurian, Llandoverian, Virginia), Kiser Lake (Silurian, Llandoverian, Ohio), and Conococheague Mountain (Ordovician, Ashgillian, Pennsylvania). A framework for investigation of the colonization of land is constructed by (1) a review of hypotheses on the origin of land plants; (2) a summary of the fossil record of terrestrial biotas; (3) an assessment of the potential of different continental depositional environments to preserve plant remains; (4) a reevaluation of Ordovician-Silurian fluvial styles based on published data; and (5) a review of pertinent data on biological soil crusts, which are considered the closest modern analogues of early terrestrial communities. -

Juniata River Water Trail Raystown Branch
Join the annual Juniata River Sojourn: www.jcwp.org Juniata River Water Trail Raystown Branch photo-JCWP (MILES CORRESPOND TO Map & Guide ALONG THE WAY– RAYSTOWN BRANCH JUNIATA RIVER WATER TRAIL RIVER MILEAGE ON MAP) This river corridor is rich in history. As you paddle down the river, Mile 73.5, Juniata Crossings Lodge and Bridge Piers- The stone lodge you’re paddling through history! “Juniata” is Native American for built in 1812, about one mile west of present day Breezewood on river left, “standing stone” or “people of the standing stone,” “Raystown” is on the National Register of Historic Sites. Overnight guests included is derived from Robert MacRay, a settler who established a trading Abraham Lincoln and Zachary Taylor. Nearby stone piers once supported a Water trails are recreational waterways on a lake, river, or ocean between specifi c post in 1750 at present-day Bedford. Prior to this, the valley was two-lane covered bridge built in 1816 (the second Juniata Crossings historic inhabitated by many peoples. bridge location) and was destroyed in the fl ood of 1936. A concrete bridge locations, containing access points and day-use and/or camping sites for the While many are familiar with the Native Americans who resided in (the third Juniata Crossings bridge location is still in use) was then built boating public. Water trails emphasize low-impact use and promote stewardship the valley, including the Shawnee, Tuscarora, Delaware (Lenape), and about 300 yards downstream from the old covered bridge, which relocated the valley’s namesake – the Juniata (Ona Jutta Hage), earlier prehistoric the present-day Lincoln Highway. -

GSA 2017 Poster Final Web
Cross Sections From The Midwest Regional Carbon Sequestration Partnership: Visualizng Subsurface Carbon Storage Opportunities Across The Central And Eastern United States Philip A. Dinterman1, Jessica Pierson Moore1, J. Eric Lewis1, Stephen F. Greb2, Kenneth G. Miller3, William J. Schmelz3 1West Virginia Geological & Economic Survey, 1 Mont Chateau Rd, Morgantown, West Virginia 26508, [email protected], 2University of Kentucky, 3Rutgers University ABSTRACT A In the fourteen years following creation of the Midwest Regional Carbon Sequestration Partnership (MRCSP) domestic energy A` production and CO2 point-source trends in the United States have experienced tremendous changes. Increasing use of natural gas as a source of electrical generation, coupled with the rise of renewable technologies, resulted in the closing of many coal-fired power plants. The coal-fired plants that remain open must now strike a balance between competitive economics and an increasingly climate-conscious public. These challenges necessitate a continued comprehensive, nation-wide, investigation of2 CO mitigation strategies, including carbon storage in deep saline aquifers as well as enhanced recovery of oil and natural gas via CO2 floods. In support of these efforts, MRCSP is characterizing Carbon Capture, Utilization, and Storage (CCUS) opportunities across a ten state-region in the central and eastern United States. Updating and packaging the existing database of petroleum fields in the region has been one of the Area of interest, with locations of multi- major MRCSP tasks of the West Virginia Geological and Economic channel seismic proles and wells & Survey (WVGES) across this region. coreholes available in region. Wells & Coreholes WVGES is also working with other MRCSP researchers to construct a set Continental Oshore Stratigraphic Test wells Individual well depth are in feet below Atlantic Section kelly bushing. -
Acid-Rock Drainage at Skytop, Centre County, Pennsylvania, 2004
U.S. Department of the Interior U.S. Geological Survey Acid-rock drainage at Skytop, Centre County, Pennsylvania, 2004 by Jane M. Hammarstrom1, Keith Brady2, and Charles A. Cravotta III3 Open-File Report 2005-1148 Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government 1 U.S. Geological Survey, 954 National Center, Reston, VA 20192 [email protected] 2 PA Department of Environmental Protection, PO Box 8461, Harrisburg, PA 17105 3 U.S. Geological Survey, 215 Limekiln Road, New Cumberland, PA 17070 Preface Construction along Interstate Highway 99 during 2003 exposed sulfidic rock within a fresh roadcut on Bald Eagle Mountain at Skytop, near State College, in Centre County, central Pennsylvania. The cut exposed pyrite veins associated with an unmined, sandstone-hosted, zinc-lead deposit. Excavated rock was crushed and used locally as road base and fill. Within months, acidic (pH<3), metal-laden seeps and surface runoff from the crushed rock piles and roadcut raised concerns about surface- and ground-water contamination and prompted a halt in road construction and the beginning of costly remediation. The Pennsylvania Department of Transportation (PennDOT) and the Pennsylvania Department of Environmental Protection expanded their site investigations and monitoring in response to the acid-drainage problem to determine the extent of the environmental effects at Skytop and to develop long-term remediation strategies. In addition, those agencies sought information on the source of the acid drainage and on the processes that led to the formation of acid-rock drainage at Skytop to assist them in selecting appropriate remediation for Skytop and in avoiding similar problems in other areas. -

Natural Resources Geology and Mineral Resources
NATURAL RESOURCES GEOLOGY AND MINERAL RESOURCES GEOLOGY the County in chronological order beginning with the youngest unit: Centre County is located within two major physiographic Allegheny Group provinces: the Appalachian Plateaus Province and the The Allegheny Group of the Pennsylvanian Ridge and Valley Province. System crops out in small areas in the northern The Appalachian Plateaus portion of the County and ranges in thickness Province is found in the west from 220 to 350 feet. The Group consists of and northwest portions of the variable layers of sandstone, shale, limestone, County and is characterized by clay and coal. (Bituminous coal has been nearly horizontal rock strata which have not extensively mined from this Group. Flint clay been deformed to any great extent by major has also been extracted from an area north of earth disturbances (tectonic forces)1. The Ridge Snow Shoe). Water is usually available in small and Valley Province is found in the south and quantities. southeast sections of the County. The angle of bedrock bedding, or dip, varies widely through Pottsville this area as result of folding. The fracturing of the rocks at the uppermost parts of the folds has The Pottsville Group of the Pennsylvanian accelerated the erosion of limestone strata. The System is comprised primarily of hard sandstone less easily eroded sandstone strata form the and forms extensive flat summits in the ridges which currently exist in the area. northwestern portion of the County. The Group includes three subdivisions, namely, Homewood Limestones and dolomites of the Cambrian and sandstone, Mercer shale and Connoquenessing Ordovician Systems are the most productive sandstone.