Predictions of Distal Radius Compressive Strength
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CS-FFRA-05 – 2005-16 Super Duty Fabricated Radius Arms NOTE
Carli Suspension: 422 Jenks Circle, Corona, CA 92880 Tech Support: (714) 532-2798 CS-FFRA-05 – 2005-16 Super Duty Fabricated Radius Arms NOTE: Please review the product instructions prior to attempting installation to ensure installer is equipped with all tools and capabilities necessary to complete the product installation. We recommend thoroughly reading the instructions at least twice prior to attempting Installation. Before beginning disassembly of the vehicle, check the “What’s Included” section of the instructions to ensure you’ve received all parts necessary to complete installation. Further, verify that the parts received are PROPER TO YOUR application (year range, motor, etc.) to avoid potential down-time in correcting potential discrepancies. Any discrepancies will be handled by Carli Suspension and the correcting products will be shipped UPS Ground. LIFETIME PRODUCT WARRANTY Carli Suspension provides a limited lifetime product warranty against defects in workmanship and materials from date of purchase to the original purchaser for all products produced by Carli Suspension. Parts not manufactured by, but made to Carli Suspension’s specifications by third party manufacturers will carry a warranty through their respective manufacturer. (i.e. King Shocks, Bilstein Shocks, Fox Shocks). Deaver Leaf Spring’s warranty will be processed by Carli Suspension. Proof of purchase (from the original purchaser only) will be required to process any warranty claims. Carli Suspension products must be purchased for the listed Retail Price reflected by the price listed on the Carli Suspension Website at the time of purchase. Carli Suspension reserves the right to refuse warranty claims made by any customer refusing or unable to present proof of purchase, or presenting proof of purchase reflecting a price lower than Carli Suspension’s Retail Price at the time the item was purchased. -

Bone Limb Upper
Shoulder Pectoral girdle (shoulder girdle) Scapula Acromioclavicular joint proximal end of Humerus Clavicle Sternoclavicular joint Bone: Upper limb - 1 Scapula Coracoid proc. 3 angles Superior Inferior Lateral 3 borders Lateral angle Medial Lateral Superior 2 surfaces 3 processes Posterior view: Acromion Right Scapula Spine Coracoid Bone: Upper limb - 2 Scapula 2 surfaces: Costal (Anterior), Posterior Posterior view: Costal (Anterior) view: Right Scapula Right Scapula Bone: Upper limb - 3 Scapula Glenoid cavity: Glenohumeral joint Lateral view: Infraglenoid tubercle Right Scapula Supraglenoid tubercle posterior anterior Bone: Upper limb - 4 Scapula Supraglenoid tubercle: long head of biceps Anterior view: brachii Right Scapula Bone: Upper limb - 5 Scapula Infraglenoid tubercle: long head of triceps brachii Anterior view: Right Scapula (with biceps brachii removed) Bone: Upper limb - 6 Posterior surface of Scapula, Right Acromion; Spine; Spinoglenoid notch Suprspinatous fossa, Infraspinatous fossa Bone: Upper limb - 7 Costal (Anterior) surface of Scapula, Right Subscapular fossa: Shallow concave surface for subscapularis Bone: Upper limb - 8 Superior border Coracoid process Suprascapular notch Suprascapular nerve Posterior view: Right Scapula Bone: Upper limb - 9 Acromial Clavicle end Sternal end S-shaped Acromial end: smaller, oval facet Sternal end: larger,quadrangular facet, with manubrium, 1st rib Conoid tubercle Trapezoid line Right Clavicle Bone: Upper limb - 10 Clavicle Conoid tubercle: inferior -
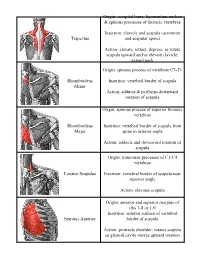
Trapezius Origin: Occipital Bone, Ligamentum Nuchae & Spinous Processes of Thoracic Vertebrae Insertion: Clavicle and Scapul
Origin: occipital bone, ligamentum nuchae & spinous processes of thoracic vertebrae Insertion: clavicle and scapula (acromion Trapezius and scapular spine) Action: elevate, retract, depress, or rotate scapula upward and/or elevate clavicle; extend neck Origin: spinous process of vertebrae C7-T1 Rhomboideus Insertion: vertebral border of scapula Minor Action: adducts & performs downward rotation of scapula Origin: spinous process of superior thoracic vertebrae Rhomboideus Insertion: vertebral border of scapula from Major spine to inferior angle Action: adducts and downward rotation of scapula Origin: transverse precesses of C1-C4 vertebrae Levator Scapulae Insertion: vertebral border of scapula near superior angle Action: elevates scapula Origin: anterior and superior margins of ribs 1-8 or 1-9 Insertion: anterior surface of vertebral Serratus Anterior border of scapula Action: protracts shoulder: rotates scapula so glenoid cavity moves upward rotation Origin: anterior surfaces and superior margins of ribs 3-5 Insertion: coracoid process of scapula Pectoralis Minor Action: depresses & protracts shoulder, rotates scapula (glenoid cavity rotates downward), elevates ribs Origin: supraspinous fossa of scapula Supraspinatus Insertion: greater tuberacle of humerus Action: abduction at the shoulder Origin: infraspinous fossa of scapula Infraspinatus Insertion: greater tubercle of humerus Action: lateral rotation at shoulder Origin: clavicle and scapula (acromion and adjacent scapular spine) Insertion: deltoid tuberosity of humerus Deltoid Action: -

Upper Extremity Fractures
Department of Rehabilitation Services Physical Therapy Standard of Care: Distal Upper Extremity Fractures Case Type / Diagnosis: This standard applies to patients who have sustained upper extremity fractures that require stabilization either surgically or non-surgically. This includes, but is not limited to: Distal Humeral Fracture 812.4 Supracondylar Humeral Fracture 812.41 Elbow Fracture 813.83 Proximal Radius/Ulna Fracture 813.0 Radial Head Fractures 813.05 Olecranon Fracture 813.01 Radial/Ulnar shaft fractures 813.1 Distal Radius Fracture 813.42 Distal Ulna Fracture 813.82 Carpal Fracture 814.01 Metacarpal Fracture 815.0 Phalanx Fractures 816.0 Forearm/Wrist Fractures Radius fractures: • Radial head (may require a prosthesis) • Midshaft radius • Distal radius (most common) Residual deformities following radius fractures include: • Loss of radial tilt (Normal non fracture average is 22-23 degrees of radial tilt.) • Dorsal angulation (normal non fracture average palmar tilt 11-12 degrees.) • Radial shortening • Distal radioulnar (DRUJ) joint involvement • Intra-articular involvement with step-offs. Step-off of as little as 1-2 mm may increase the risk of post-traumatic arthritis. 1 Standard of Care: Distal Upper Extremity Fractures Copyright © 2007 The Brigham and Women's Hospital, Inc. Department of Rehabilitation Services. All rights reserved. Types of distal radius fracture include: • Colle’s (Dinner Fork Deformity) -- Mechanism: fall on an outstretched hand (FOOSH) with radial shortening, dorsal tilt of the distal fragment. The ulnar styloid may or may not be fractured. • Smith’s (Garden Spade Deformity) -- Mechanism: fall backward on a supinated, dorsiflexed wrist, the distal fragment displaces volarly. • Barton’s -- Mechanism: direct blow to the carpus or wrist. -
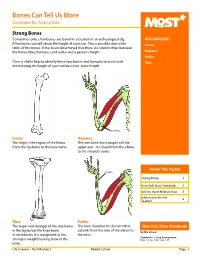
Bones Can Tell Us More Compiled By: Nancy Volk
Bones Can Tell Us More Compiled By: Nancy Volk Strong Bones Sometimes only a few bones are found in a location in an archeological dig. VOCABULARY A few bones can tell about the height of a person. This is possible due to the Femur ratios of the bones. It has been determined that there are relationships between the femur, tibia, humerus, and radius and a person’s height. Humerus Radius Here is a little help to identify these four bones and formulas to assist with Tibia determining the height of a person based on bone length. Humerus Femur: Humerus: The thigh is the region of the femur. The arm bone most people call the From the hip bone to the knee bone. upper arm. It is found from the elbow to the shoulder joints. Inside This Packet Radius Strong Bones 1 New York State Standards 1 Activity: Bone Relationships 2 Information for the Teacher 4 Tibia: Radius: The larger and stronger of the two bones The bone found in the forearm that New York State Standards in the leg below the knee bone. extends from the side of the elbow to Middle School In vertebrates It is recognized as the the wrist. Standard 4: Living Environment strongest weight bearing bone in the Idea 1: 1.2a, 1.2b, 1.2e, 1.2f body. Life Sciences - Post Module 3 Middle School Page 1 Activity: Bone Relationships MATERIALS NEEDED Skeleton Formulas: Tape Measure Bone relationship is represented by the following formulas: Directions and formulas P represents the person’s height. The last letter of each formula stands for the Calculator known length of the bone (femur, tibia, humerus, or radius) through measurement. -

Distal Radius Fracture
Distal Radius Fracture Osteoporosis, a common condition where bones become brittle, increases the risk of a wrist fracture if you fall. How are distal radius fractures diagnosed? Your provider will take a detailed health history and perform a physical evaluation. X-rays will be taken to confirm a fracture and help determine a treatment plan. Sometimes an MRI or CT scan is needed to get better detail of the fracture or to look for associated What is a distal radius fracture? injuries to soft tissues such as ligaments or Distal radius fracture is the medical term for tendons. a “broken wrist.” To fracture a bone means it is broken. A distal radius fracture occurs What is the treatment for distal when a sudden force causes the radius bone, radius fracture? located on the thumb side of the wrist, to break. The wrist joint includes many bones Treatment depends on the severity of your and joints. The most commonly broken bone fracture. Many factors influence treatment in the wrist is the radius bone. – whether the fracture is displaced or non-displaced, stable or unstable. Other Fractures may be closed or open considerations include age, overall health, (compound). An open fracture means a bone hand dominance, work and leisure activities, fragment has broken through the skin. There prior injuries, arthritis, and any other injuries is a risk of infection with an open fracture. associated with the fracture. Your provider will help determine the best treatment plan What causes a distal radius for your specific injury. fracture? Signs and Symptoms The most common cause of distal radius fracture is a fall onto an outstretched hand, • Swelling and/or bruising at the wrist from either slipping or tripping. -

Conservative Vs. Surgical Management of Ulnar Styloid Fractures Associated with Distal Radius Fractures
CLINICAL RESEARCH Conservative vs. surgical management of ulnar styloid fractures associated with distal radius fractures Cristian Robles, Santiago Iglesias, Christian Allende Nores, Pablo Rotella, Martín Caloia, Miguel Capomassi Orthopedics and Traumatology Department, Sanatorio Allende (Córdoba, Argentina) ABSTRACT Objectives: To evaluate potential differences in clinical and radiological outcomes after surgical versus conservative management of ulnar styloid fractures associated with unstable distal radius fractures treated by locked volar plating. Materials and Methods: This was a multicenter, retrospective and descriptive study including surgical patients treated at four different institutions between 2009 and 2012 for ulnar styloid fractures associated with unstable distal radius fractures. Ulnar styloid fractures were treated con- servatively in group I and surgically in group II. Results: The average follow-up was 56 months. The study included 57 patients divided into two groups (group I [29 cases] and group II [28 cases]). Patients in group II had 2.76 times (95% CI: 1.086; 8.80) more chances of achieving bone union than those in group I. DASH and pain scores, both at rest and during activity, did not show significant differences between the two groups (p = 0.276 and p = 0.877). Group I presented milder ulnar deviation and better strength (p = 0.0194 and p = 0.024). Conclusions: Although patients who underwent surgery for ulnar styloid fractures had 2.76 more chances of achieving bone union than those who received conservative management, there were no significant differences between both groups in subjective evaluations (DASH and pain scores) or when considering the degree of ulnar styloid involve- ment. However, the parameters of strength and ulnar deviation were better in the conservative management group. -

17 Radial Styloidectomy David M
17 Radial Styloidectomy David M. Kalainov, Mark S. Cohen, and Stephanie Sweet xcision of the radial styloid gained recognition osteotomy removed 92% of the radioscaphocapitate in 1948 when Barnard and Stubbins1 reported on and 21% of the long radiolunate ligament origins. The Eten scaphoid fracture nonunions treated with transverse osteotomy was the most invasive, detach- bone grafting and radial styloidectomy. The procedure ing 95% of the radioscaphocapitate and 46% of the has since been advocated to address radioscaphoid long radiolunate ligament origins. arthritis developing from a variety of injuries, includ- In another cadaveric model, Nakamura et al19 ex- ing previous fractures of the radial styloid and scaphoid, amined the effects of increasingly larger oblique sty- and arthritis related to posttraumatic scapholunate loidectomies on carpal stability. They concluded that instability.2–8 the procedure should be limited to a 3- to 4-mm bony Resection of the radial styloid has also been a use- resection. With axial loading, significantly increased ful adjunct to other procedures where there is poten- radial, ulnar, and palmar displacements of the carpus tial for impingement between the styloid process and were detected after removing 6-mm and 10-mm sty- distal scaphoid or trapezium.9–15 Authors have in- loid segments. The 6-mm cut violated the radio- cluded discussion of successful radial styloidectomy scaphocapitate ligament origin, whereas the 10-mm in descriptions of proximal row carpectomy, mid- cut removed the radioscaphocapitate and a portion of carpal arthrodesis, and triscaphe fusion procedures. the long radiolunate ligament origins. Only an in- On occasion, an individual may be too physically un- significant change in carpal translation was detected fit or unwilling to undergo an extensive operation to after a 3-mm osteotomy. -

The Anatomy of the Bicipital Tuberosity and Distal Biceps Tendon
The anatomy of the bicipital tuberosity and distal biceps tendon Augustus D. Mazzocca, MD,a Mark Cohen, MD,b Eric Berkson, MD,b Gregory Nicholson, MD,b Bradley C. Carofino, MD,a Robert Arciero, MD,a and Anthony A. Romeo, MDb Farmington, CT, and Chicago, IL The anatomy of the distal biceps tendon and bicipi- and the mean BT-radial styloid angle is 123° Ϯ 10°. tal tuberosity (BT) is important in the pathophysiol- None of the measurements correlated with patient ogy of tendon rupture, as well as surgical repair. age, sex, or race. We concluded that the morphology Understanding the dimensions of the BT and its an- of the BT ridge is variable. The insertion footprint of gular relationship to the radial head and radial the distal biceps tendon is on the ulnar aspect of the styloid will facilitate surgical procedures such as re- BT ridge. The dimensions of the radius and BT are ap- construction of the distal biceps tendon, radial head plicable to several surgical procedures about the el- prosthesis implantation, and reconstruction of proxi- bow. (J Shoulder Elbow Surg 2007;16:122-127.) mal radius trauma. We examined 178 dried cadav- eric radii, and the following measurements were col- The recognition and treatment of distal biceps ten- lected: radial length, length and width of the BT, don ruptures have increased over time. Previously, diameter of the radius just distal to the BT, distance this injury was considered rare; only 65 cases were from the radial head to the BT, radial head diame- reported before 1941.2,4 However, a more recent ter, width of the radius at the BT, radial neck-shaft retrospective study identified the incidence to be 1.2 10 angle, and styloid angle. -

Ford Super Duty 2-1/2” Radius Arm Kit
Part#: 013261-013262 Ford Super Duty 2-1/2” Radius Arm Kit Ford F-250, F350 | 2017-2019 Rev.012920 491 W. Garfield Ave., Coldwater, MI 49036 . Phone: 517-279-2135 Web: www.bds-suspension.com • E-mail: [email protected] Read And Understand All Instructions And Warnings Prior To Installation Of System And Operation Of Vehicle. Your truck is about to be fitted with the best suspension system on the market today. That means you will be driving the baddest looking truck in the neighborhood, and you’ll have the warranty to ensure that it stays that way for years to come. Thank you for choosing BDS Suspension! BEFORE YOU START BDS Suspension Co. recommends this system be installed by a professional technician. In addition to these instructions, professional knowledge of disassembly/ reassembly procedures and post installation checks must be known. FOR YOUR SAFETY Certain BDS Suspension products are intended to improve off-road performance. Modifying your vehicle for off-road use may result in the vehicle handling differently than a factory equipped vehicle. Extreme care must be used to prevent loss of control or vehicle rollover. Failure to drive your modified vehicle safely may result in serious injury or death. BDS Suspension Co. does not recommend the combined use of suspension lifts, body lifts, or other lifting devices. You should never operate your modified vehicle under the influence of alcohol or drugs. Always drive your modified 35x12.50x17(18)(20) Tire vehicle at reduced speeds to ensure your ability to control your vehicle under 4-1/2” ~ 5” Backspace Wheel all driving conditions. -

Effect of Ulnar Styloid Fracture on Functional Outcome of Colle's Fractures
International Surgery Journal Dar IH et al. Int Surg J. 2015 Nov;2(4):556-559 http://www.ijsurgery.com pISSN 2349-3305 | eISSN 2349-2902 DOI: http://dx.doi.org/10.18203/2349-2902.isj20151079 Research Article Effect of ulnar styloid fracture on functional outcome of Colle’s fractures: a comparative analysis of two groups Imtiyaz Hussain Dar1, Iftikhar H. Wani1*, Ummar Mumtaz1, Masrat Jan2 1Department of Orthopedics, Government Medical College Hospital for Bone and Joint Surgery, Srinagar-190005, Kashmir, India 2 Department of Anesthesia and Intensive Care, Government Medical College, Srinagar-190005, Kashmir, India Received: 21 July 2015 Accepted: 24 August 2015 *Correspondence: Dr. Iftikhar H. Wani, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: The negative impact of ulnar sided injuries on distal radius fractures has opened another field of research and is gaining more attention. The aim of our study is to assess the impact of ulnar styloid process fracture on functional outcome of distal radius fractures managed conservatively. Methods: Radiological and Medical records of 150 patients of distal radius with and without styloid process fractures were retrospectively reviewed. Fractures were classified as per Frykman’s classification and patients were assessed for pain, grip and range of motion in addition to other parameters in Mayo’s wrist score. The instability at distal radioulnar joint was evaluated and compared in two groups. -

A STUDY of MORPHOLOGY and MORPHOMETRY of PROXIMAL END of DRY RADIUS BONES with ITS CLINICAL IMPLICATIONS Suraj Ethiraj 1, Jyothi K C *2, Shailaja Shetty 3
International Journal of Anatomy and Research, Int J Anat Res 2019, Vol 7(3.1):6712-16. ISSN 2321-4287 Original Research Article DOI: https://dx.doi.org/10.16965/ijar.2019.203 A STUDY OF MORPHOLOGY AND MORPHOMETRY OF PROXIMAL END OF DRY RADIUS BONES WITH ITS CLINICAL IMPLICATIONS Suraj Ethiraj 1, Jyothi K C *2, Shailaja Shetty 3. 1 Final year MBBS, M S Ramaiah Medical College, Bangalore, Karnataka, India. 2 Assistant Professor, Department of Anatomy, M S Ramaiah Medical College, Bangalore, Karnataka, India. 3 Professor & HOD, Department of Anatomy, M S Ramaiah Medical College, Bangalore, Karnataka, India. ABSTRACT Background: Fracture of the radial head constitute 1/3rd of all the elbow fractures. It occurs as a result of a fall on an outstretched hand or a direct blow to the lateral aspect of elbow joint. This is now becoming more common due to pre existing co-morbidities like osteoporosis and chronic osteoarthritis. Surgical correction of the comminuted fractures of radial head involves reconstruction or replacement with artificial radial head prosthesis in cases where reconstruction is not possible. Aims and Objectives: To analyze the morphometric details of proximal end of radius and to describe the morphological features of head and bicipital tuberosity of the radius. Materials & Methodology: Sixty dry human adult radius bones of unknown age and sex were assessed for morphometric and morphological characters. Vernier caliper was used to measure the various parameters on the proximal ends of radius bones. The data was tabulated and analyzed using SPSS software. Results: The mean length of radius was found to be 23.98 cm.