SOUTH RUPUNUNI BIODIVERSITY ASSESSMENT TEAM (BAT) EXPEDITION October 22 – November 7, 2013
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Web-Book Catalog 2021-05-10
Lehigh Gap Nature Center Library Book Catalog Title Year Author(s) Publisher Keywords Keywords Catalog No. National Geographic, Washington, 100 best pictures. 2001 National Geogrpahic. Photographs. 779 DC Miller, Jeffrey C., and Daniel H. 100 butterflies and moths : portraits from Belknap Press of Harvard University Butterflies - Costa 2007 Janzen, and Winifred Moths - Costa Rica 595.789097286 th tropical forests of Costa Rica Press, Cambridge, MA rica Hallwachs. Miller, Jeffery C., and Daniel H. 100 caterpillars : portraits from the Belknap Press of Harvard University Caterpillars - Costa 2006 Janzen, and Winifred 595.781 tropical forests of Costa Rica Press, Cambridge, MA Rica Hallwachs 100 plants to feed the bees : provide a 2016 Lee-Mader, Eric, et al. Storey Publishing, North Adams, MA Bees. Pollination 635.9676 healthy habitat to help pollinators thrive Klots, Alexander B., and Elsie 1001 answers to questions about insects 1961 Grosset & Dunlap, New York, NY Insects 595.7 B. Klots Cruickshank, Allan D., and Dodd, Mead, and Company, New 1001 questions answered about birds 1958 Birds 598 Helen Cruickshank York, NY Currie, Philip J. and Eva B. 101 Questions About Dinosaurs 1996 Dover Publications, Inc., Mineola, NY Reptiles Dinosaurs 567.91 Koppelhus Dover Publications, Inc., Mineola, N. 101 Questions About the Seashore 1997 Barlowe, Sy Seashore 577.51 Y. Gardening to attract 101 ways to help birds 2006 Erickson, Laura. Stackpole Books, Mechanicsburg, PA Birds - Conservation. 639.978 birds. Sharpe, Grant, and Wenonah University of Wisconsin Press, 101 wildflowers of Arcadia National Park 1963 581.769909741 Sharpe Madison, WI 1300 real and fanciful animals : from Animals, Mythical in 1998 Merian, Matthaus Dover Publications, Mineola, NY Animals in art 769.432 seventeenth-century engravings. -

Phylogenetic Relationships Within the Speciose Family Characidae
Oliveira et al. BMC Evolutionary Biology 2011, 11:275 http://www.biomedcentral.com/1471-2148/11/275 RESEARCH ARTICLE Open Access Phylogenetic relationships within the speciose family Characidae (Teleostei: Ostariophysi: Characiformes) based on multilocus analysis and extensive ingroup sampling Claudio Oliveira1*, Gleisy S Avelino1, Kelly T Abe1, Tatiane C Mariguela1, Ricardo C Benine1, Guillermo Ortí2, Richard P Vari3 and Ricardo M Corrêa e Castro4 Abstract Background: With nearly 1,100 species, the fish family Characidae represents more than half of the species of Characiformes, and is a key component of Neotropical freshwater ecosystems. The composition, phylogeny, and classification of Characidae is currently uncertain, despite significant efforts based on analysis of morphological and molecular data. No consensus about the monophyly of this group or its position within the order Characiformes has been reached, challenged by the fact that many key studies to date have non-overlapping taxonomic representation and focus only on subsets of this diversity. Results: In the present study we propose a new definition of the family Characidae and a hypothesis of relationships for the Characiformes based on phylogenetic analysis of DNA sequences of two mitochondrial and three nuclear genes (4,680 base pairs). The sequences were obtained from 211 samples representing 166 genera distributed among all 18 recognized families in the order Characiformes, all 14 recognized subfamilies in the Characidae, plus 56 of the genera so far considered incertae sedis in the Characidae. The phylogeny obtained is robust, with most lineages significantly supported by posterior probabilities in Bayesian analysis, and high bootstrap values from maximum likelihood and parsimony analyses. -

Furness, Mcdiarmid, Heyer, Zug.Indd
south american Journal of Herpetology, 5(1), 2010, 13-29 © 2010 brazilian society of herpetology Oviduct MOdificatiOns in fOaM-nesting frOgs, with eMphasis On the genus LeptodactyLus (aMphibia, LeptOdactyLidae) Andrew I. Furness1, roy w. McdIArMId2, w. ronAld Heyer3,5, And GeorGe r. ZuG4 1 department of Biology, university of california, Riverside, ca 92501, usa. e‑mail: [email protected] 2 us Geological survey, patuxent Wildlife Research center, National Museum of Natural History, MRc 111, po Box 37012, smithsonian Institution, Washington, dc 20013‑7012, usa. e‑mail: [email protected] 3 National Museum of Natural History, MRc 162, po Box 37012, smithsonian Institution, Washington, dc 20013‑7012. e‑mail: [email protected] 4 National Museum of Natural History, MRc 162, po Box 37012, smithsonian Institution, Washington, dc 20013‑7012. e‑mail: [email protected] 5 corresponding author. AbstrAct. various species of frogs produce foam nests that hold their eggs during development. we examined the external morphology and histology of structures associated with foam nest production in frogs of the genus Leptodactylus and a few other taxa. we found that the posterior convolutions of the oviducts in all mature female foam-nesting frogs that we examined were enlarged and compressed into globular structures. this organ-like portion of the oviduct has been called a “foam gland” and these structures almost certainly produce the secretion that is beaten by rhythmic limb movements into foam that forms the nest. however, the label “foam gland” is a misnomer because the structures are simply enlarged and tightly folded regions of the pars convoluta of the oviduct, rather than a separate structure; we suggest the name pars convoluta dilata (pcd) for this feature. -

A New Computing Environment for Modeling Species Distribution
EXPLORATORY RESEARCH RECOGNIZED WORLDWIDE Botany, ecology, zoology, plant and animal genetics. In these and other sub-areas of Biological Sciences, Brazilian scientists contributed with results recognized worldwide. FAPESP,São Paulo Research Foundation, is one of the main Brazilian agencies for the promotion of research.The foundation supports the training of human resources and the consolidation and expansion of research in the state of São Paulo. Thematic Projects are research projects that aim at world class results, usually gathering multidisciplinary teams around a major theme. Because of their exploratory nature, the projects can have a duration of up to five years. SCIENTIFIC OPPORTUNITIES IN SÃO PAULO,BRAZIL Brazil is one of the four main emerging nations. More than ten thousand doctorate level scientists are formed yearly and the country ranks 13th in the number of scientific papers published. The State of São Paulo, with 40 million people and 34% of Brazil’s GNP responds for 52% of the science created in Brazil.The state hosts important universities like the University of São Paulo (USP) and the State University of Campinas (Unicamp), the growing São Paulo State University (UNESP), Federal University of São Paulo (UNIFESP), Federal University of ABC (ABC is a metropolitan region in São Paulo), Federal University of São Carlos, the Aeronautics Technology Institute (ITA) and the National Space Research Institute (INPE). Universities in the state of São Paulo have strong graduate programs: the University of São Paulo forms two thousand doctorates every year, the State University of Campinas forms eight hundred and the University of the State of São Paulo six hundred. -

TOUR REPORT Southwestern Amazonia 2017 Final
For the first time on a Birdquest tour, the Holy Grail from the Brazilian Amazon, Rondonia Bushbird – male (Eduardo Patrial) BRAZIL’S SOUTHWESTERN AMAZONIA 7 / 11 - 24 JUNE 2017 LEADER: EDUARDO PATRIAL What an impressive and rewarding tour it was this inaugural Brazil’s Southwestern Amazonia. Sixteen days of fine Amazonian birding, exploring some of the most fascinating forests and campina habitats in three different Brazilian states: Rondonia, Amazonas and Acre. We recorded over five hundred species (536) with the exquisite taste of specialties from the Rondonia and Inambari endemism centres, respectively east bank and west bank of Rio Madeira. At least eight Birdquest lifer birds were acquired on this tour: the rare Rondonia Bushbird; Brazilian endemics White-breasted Antbird, Manicore Warbling Antbird, Aripuana Antwren and Chico’s Tyrannulet; also Buff-cheeked Tody-Flycatcher, Acre Tody-Tyrant and the amazing Rufous Twistwing. Our itinerary definitely put together one of the finest selections of Amazonian avifauna, though for a next trip there are probably few adjustments to be done. The pre-tour extension campsite brings you to very basic camping conditions, with company of some mosquitoes and relentless heat, but certainly a remarkable site for birding, the Igarapé São João really provided an amazing experience. All other sites 1 BirdQuest Tour Report: Brazil’s Southwestern Amazonia 2017 www.birdquest-tours.com visited on main tour provided considerably easy and very good birding. From the rich east part of Rondonia, the fascinating savannas and endless forests around Humaitá in Amazonas, and finally the impressive bamboo forest at Rio Branco in Acre, this tour focused the endemics from both sides of the medium Rio Madeira. -

Biodiversity of the Southern Rupununi Savannah World Wildlife Fund and Global Wildlife Conservation
THIS REPORT HAS BEEN PRODUCED IN GUIANAS COLLABORATION VERZICHT APERWITH: Ç 2016 Biodiversity of the Southern Rupununi Savannah World Wildlife Fund and Global Wildlife Conservation 2016 WWF-Guianas Global Wildlife Conservation Guyana Office PO Box 129 285 Irving Street, Queenstown Austin, TX 78767 USA Georgetown, Guyana [email protected] www.wwfguianas.org [email protected] Text: Juliana Persaud, WWF-Guianas, Guyana Office Concept: Francesca Masoero, WWF-Guianas, Guyana Office Design: Sita Sugrim for Kriti Review: Brian O’Shea, Deirdre Jaferally and Indranee Roopsind Map: Oronde Drakes Front cover photos (left to right): Rupununi Savannah © Zach Montes, Giant Ant Eater © Gerard Perreira, Red Siskin © Meshach Pierre, Jaguar © Evi Paemelaere. Inside cover photo: Gallery Forest © Andrew Snyder. OF BIODIVERSITYTHE SOUTHERN RUPUNUNI SAVANNAH. Guyana-South America. World Wildlife Fund and Global Wildlife Conservation 2016 This booklet has been produced and published thanks to: 1 WWF Biodiversity Assessment Team Expedition Southern Rupununi - Guyana. The Southern Rupununi Biodiversity Survey Team / © WWF - GWC. Biodiversity Assessment Team (BAT) Survey. This programme was created by WWF-Guianas in 2013 to contribute to sound land- use planning by filling biodiversity data gaps in critical areas in the Guianas. As far as possible, it also attempts to understand the local context of biodiversity use and the potential threats in order to recommend holistic conservation strategies. The programme brings together local knowledge experts and international scientists to assess priority areas. With each BAT Survey, species new to science or new country records are being discovered. This booklet acknowledges the findings of a BAT Survey carried out during October-November 2013 in the southern Rupununi savannah, at two locations: Kusad Mountain and Parabara. -
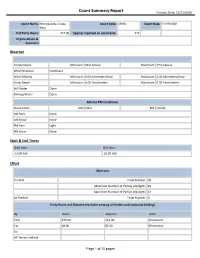
Count Summary Report Printout Date: 11/11/2016
Count Summary Report Printout Date: 11/11/2016 Count Name: Monteverde, Costa Count Code: CRMO Count Date: 12/19/2001 Rica # of Party Hours: 257.00 Species reported on count date: 376 Organizations & Sponsors: Weather Temperature Minimum: 59.0 Celsius Maximum: 77.0 Celsius Wind Direction Northeast Wind Velocity Minimum: 0.00 Kilometers/hour Maximum: 6.00 Kilometers/hour Snow Depth Minimum: 0.00 Centimeters Maximum: 0.00 Centimeters Still Water Open Moving Water Open AM and PM Conditions Cloud Cover AM: Clear PM: Cloudy AM Rain None AM Snow None PM Rain Light PM Snow None Start & End Times Start time End time 12:00 AM 10:00 AM Effort Observers In Field Total Number: 70 Minimum Number of Parties (daylight): 19 Maximum Number of Parties (daylight): 24 At Feeders Total Number: 0 Party Hours and Distance (excludes viewing at feeders and nocturnal birding) By Hours Distance Units Foot 239.00 114.00 Kilometers Car 18.00 85.00 Kilometers Air All-Terrain Vehicle Page 1 of 12 pages Count Summary Report Printout Date: 11/11/2016 Bicycle Dog Sled Golfcart Horseback Motorized Boat Non-Motorized Boat Skis/Xc-Skis Snowmachine Snowshoe Wheelchair Other Time and Distance Hours Distance Units At Feeders 0.00 Nocturnal Birding 17.00 26.00 Miles Total Party 257.00 199.00 Kilometers Checklist Species Number Number/Party Hrs. Flags Editorial Codes Highland Tinamou 3 0.0117 Great Tinamou 2 0.0078 Gray-headed Chachalaca 33 0.1284 Crested Guan 20 0.0778 Black Guan 18 0.0700 Black-breasted Wood-Quail 56 0.2179 Least Grebe 5 0.0195 Anhinga 1 0.0039 Fasciated Tiger-Heron -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Provisional List of Birds of the Rio Tahuauyo Areas, Loreto, Peru
Provisional List of Birds of the Rio Tahuauyo areas, Loreto, Peru Compiled by Carol R. Foss, Ph.D. and Josias Tello Huanaquiri, Guide Status based on expeditions from Tahuayo Logde and Amazonia Research Center TINAMIFORMES: Tinamidae 1. Great Tinamou Tinamus major 2. White- throated Tinamou Tinamus guttatus 3. Cinereous Tinamou Crypturellus cinereus 4. Little Tinamou Crypturellus soui 5. Undulated Tinamou Crypturellus undulates 6. Variegated Tinamou Crypturellus variegatus 7. Bartlett’s Tinamou Crypturellus bartletti ANSERIFORMES: Anhimidae 8. Horned Screamer Anhima cornuta ANSERIFORMES: Anatidae 9. Muscovy Duck Cairina moschata 10. Blue-winged Teal Anas discors 11. Masked Duck Nomonyx dominicus GALLIFORMES: Cracidae 12. Spix’s Guan Penelope jacquacu 13. Blue-throated Piping-Guan Pipile cumanensis 14. Speckled Chachalaca Ortalis guttata 15. Wattled Curassow Crax globulosa 16. Razor-billed Curassow Mitu tuberosum GALLIFORMES: Odontophoridae 17. Marbled Wood-Quall Odontophorus gujanensis 18. Starred Wood-Quall Odontophorus stellatus PELECANIFORMES: Phalacrocoracidae 19. Neotropic Cormorant Phalacrocorax brasilianus PELECANIFORMES: Anhingidae 20. Anhinga Anhinga anhinga CICONIIFORMES: Ardeidae 21. Rufescent Tiger-Heron Tigrisoma lineatum 22. Agami Heron Agamia agami 23. Boat-billed Heron Cochlearius cochlearius 24. Zigzag Heron Zebrilus undulatus 25. Black-crowned Night-Heron Nycticorax nycticorax 26. Striated Heron Butorides striata 27. Cattle Egret Bubulcus ibis 28. Cocoi Heron Ardea cocoi 29. Great Egret Ardea alba 30. Cappet Heron Pilherodius pileatus 31. Snowy Egret Egretta thula 32. Little Blue Heron Egretta caerulea CICONIIFORMES: Threskiornithidae 33. Green Ibis Mesembrinibis cayennensis 34. Roseate Spoonbill Platalea ajaja CICONIIFORMES: Ciconiidae 35. Jabiru Jabiru mycteria 36. Wood Stork Mycteria Americana CICONIIFORMES: Cathartidae 37. Turkey Vulture Cathartes aura 38. Lesser Yellow-headed Vulture Cathartes burrovianus 39. -

Colombia 1 000 Birds Mega Tour II 21St November to 19Th December 2014 (29 Days)
Colombia 1 000 Birds Mega Tour II 21st November to 19th December 2014 (29 days) Lance-tailed Manakin by Dennis Braddy Trip report compiled by Tour Leader: Rob Williams Trip Report - RBT Colombia Mega II 2014 2 An early start on day 1 saw us heading to Mundo Nuevo. Our first stop en route produced a flurry of birds including Northern Mountain Cacique, Golden-fronted Whitestart, Barred Becard, Mountain Elaenia and a Green-tailed Trainbearer feeding young at a nest. We continued up to the altitude where the endemic Flame-winged Parakeets breed and breakfasted while we awaited them. We were rewarded with great scope looks at this threatened species. The area also gave us a flurry of other birds including Scarlet-bellied Mountain Tanager, Rufous-breasted Chat- Tyrant, Pearled Treerunner and Smoke-coloured Pewee. We continued up to the edge of the paramo and birded a track inside Chingaza National Park. Activity was low but we persisted and were rewarded with a scattering of birds including Glossy, Masked and Bluish Flowerpiercers, Slaty Brush Finch, Glowing and Coppery-bellied Pufflegs, and Brown-backed Chat-Tyrant. The endemic Bronze-tailed Thornbill only gave frustrating brief flyby views. Great looks however were had of the endemic Pale-bellied Tapaculo, singing from surprisingly high up in a bush. The track back down gave us Rufous Wren, Superciliated and Black-capped Hemispingus and Tourmaline Sunangel. Further down the road a Buff- breasted Mountain Tanager and some Beryl-spangled Tanagers were found before we headed back to La Calera. After lunch in a local restaurant we headed to the Siecha gravel pits. -

The Edgar Mittelholzer Memorial Lectures
BEACONS OF EXCELLENCE: THE EDGAR MITTELHOLZER MEMORIAL LECTURES VOLUME 3: 1986-2013 Edited and with an Introduction by Andrew O. Lindsay 1 Edited by Andrew O. Lindsay BEACONS OF EXCELLENCE: THE EDGAR MITTELHOLZER MEMORIAL LECTURES - VOLUME 3: 1986-2013 Preface © Andrew Jefferson-Miles, 2014 Introduction © Andrew O. Lindsay, 2014 Cover design by Peepal Tree Press Cover photograph: Courtesy of Jacqueline Ward All rights reserved No part of this publication may be reproduced or transmitted in any form without permission. Published by the Caribbean Press. ISBN 978-1-907493-67-6 2 Contents: Tenth Series, 1986: The Arawak Language in Guyanese Culture by John Peter Bennett FOREWORD by Denis Williams .......................................... 3 PREFACE ................................................................................. 5 THE NAMING OF COASTAL GUYANA .......................... 7 ARAWAK SUBSISTENCE AND GUYANESE CULTURE ........................................................................ 14 Eleventh Series, 1987. The Relevance of Myth by George P. Mentore PREFACE ............................................................................... 27 MYTHIC DISCOURSE......................................................... 29 SOCIETY IN SHODEWIKE ................................................ 35 THE SELF CONSTRUCTED ............................................... 43 REFERENCES ....................................................................... 51 Twelfth Series, 1997: Language and National Unity by Richard Allsopp CHAIRMAN’S FOREWORD -

Eagle-Eye Tours Guyana Tour Species List January 17-29, 2019
Guyana Tour Species List Tour Leader: Paul Prior Eagle-Eye Tours January 17-29, 2019 BIRD SPECIES Seen/ Common Name Scientific Name Heard TINAMOUS 1 Great Tinamou Tinamus major H 2 Cinereous Tinamou Crypturellus cinereus H 3 Little Tinamou Crypturellus soui H 4 Undulated Tinamou Crypturellus undulatus H 5 Red-legged Tinamou Crypturellus erythropus H 6 Variegated Tinamou Crypturellus variegatus H DUCKS, GEESE, AND WATERFOWL 7 White-faced Whistling-Duck Dendrocygna viduata S 8 Muscovy Duck Cairina moschata S 9 Masked Duck Nomonyx dominicus S GUANS, CHACHALACAS, AND CURASSOWS 10 Variable Chachalaca Ortalis motmot S 11 Marail Guan Penelope marail S 12 Spix's Guan Penelope jacquacu S 13 Black Curassow Crax alector S NEW WORLD QUAIL 14 Crested Bobwhite Colinus cristatus S FLAMINGOS 15 American Flamingo Phoenicopterus ruber S GREBES 16 Least Grebe Tachybaptus dominicus S 17 Pied-billed Grebe Podilymbus podiceps S STORKS 18 Maguari Stork Ciconia maguari S 19 Jabiru Jabiru mycteria S 20 Wood Stork Mycteria americana S FRIGATEBIRDS 21 Magnificent Frigatebird Fregata magnificens S CORMORANTS AND SHAGS 22 Neotropic Cormorant Phalacrocorax brasilianus S ANHINGAS 23 Anhinga Anhinga anhinga S PELICANS 24 Brown Pelican Pelecanus occidentalis S HERONS, EGRETS, AND BITTERNS Page1 of 15 Guyana Tour Species List Tour Leader: Paul Prior Eagle-Eye Tours January 17-29, 2019 BIRD SPECIES Seen/ Common Name Scientific Name Heard 25 Pinnated bittern Botaurus pinnatus S 26 Cocoi Heron Ardea cocoi S 27 Great Egret Ardea alba S 28 Snowy Egret Egretta thula S 29 Little