Geology and Mineralogy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Geology of the Middle Precambrian Rove Formation in Northeastern Minnesota
MINNESOTA GEOLOGICAL SURVEY 5 P -7 Special Publication Series The Geology of the Middle Precambrian Rove Formation in northeastern Minnesota G. B. Morey UNIVERSITY OF MINNESOTA MINNEAPOLIS • 1969 I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I THE GEOLOGY OF THE MIDDLE PRECAMBRIAN ROVE FORMATION IN NORTHEASTERN MINNESOTA by G. B. Morey CONTENTS Page Abstract ........................................... 1 Introduction. 3 Location and scope of study. 3 Acknowledgements .. 3 Regional geology . 5 Structural geology . 8 Rock nomenclature . 8 Stratigraphy . .. 11 Introduction . .. 11 Nomenclature and correlation. .. 11 Type section . .. 11 Thickness . .. .. 14 Lower argillite unit. .. 16 Definition, distribution, and thickness. .. 16 Lithologic character . .. 16 Limestones. .. 17 Concretions. .. 17 Transition unit . .. 17 Definition, distribution, and thickness. .. 17 Lithologic character . .. 19 Thin-bedded graywacke unit . .. 19 Definition, distribution, and thickness. .. 19 Lithologic character. .. 20 Concretions ... .. 20 Sedimentary structures. .. 22 Internal bedding structures. .. 22 Structureless bedding . .. 23 Laminated bedding . .. 23 Graded bedding. .. 23 Cross-bedding . .. 25 Convolute bedding. .. 26 Internal bedding sequences . .. 26 Post-deposition soft sediment deformation structures. .. 27 Bed pull-aparts . .. 27 Clastic dikes . .. 27 Load pockets .. .. 28 Flame structures . .. 28 Overfolds . .. 28 Microfaults. .. 28 Ripple marks .................................. 28 Sole marks . .. 28 Groove casts . .. 30 Flute casts . -

How Environmentally Responsible Mining Will Boost Minnesota's Economy
Unearthing Prosperity How Environmentally Responsible Mining Will Boost Minnesota’s Economy ISAAC M. ORR, DEBRA W. STRUHSACKER, JOHN PHELAN Isaac Orr is a policy fellow at Center of the American Experiment specializing in energy and environmental policy. He graduated from the University of Wisconsin–Eau Claire with studies in political science and geology, winning awards for his undergraduate geology research. Debra Struhsacker is a hardrock mining policy expert with over 30 years of hands-on expertise with the environmental and public land laws and regulations pertaining to mineral exploration and mine development. Ms. Struhsacker is a Phi Beta Kappa graduate of Wellesley College where she majored in both geology and French. She also has a Master of Science degree in geology from the University of Montana. Debra is one of the founders of the Women’s Mining Coalition and currently serves on the Coalition’s Board of Directors. She is a Certified Professional Geologist with the American Institute of Professional Geologists. Her professional memberships include the Mining and Metallurgical Society of America; the Society for Mining, Metallurgy, and Exploration, Inc.; and the Geological Society of Nevada. She has served twice as a trustee of the Northwest Mining Association (now the American Exploration & Mining Association) and is an Emeritus Member of the Board of Directors of the Mountain States Legal Foundation. John Phelan is an economist at Center of the American Experiment. He is a graduate of Birkbeck College, University of London, where he earned a BSc in Economics, and the London School of Economics where he earned an MSc. John worked in finance for ten years before becoming a professional economist. -

The Mineral Industry of Minnesota
MINNESOTA KITTSON ROSEAU SG LAKE OF THE WOODS CS MARSHALL Peat KOOCHICHING PENNINGTON BELTRAMI Peat COOK E Lime RED LAK CS ST LOUIS SG D-G CLEAR Fe POLK 1 WATER LAKE ITASCA SG CS Fe CS NORMAN CS Peat SG MAHNOMEN SG SG HUBBARD CASS 2 SG Peat SG Lime BECKER SG Peat CLAY LEGEND AITKIN County boundary Peat Peat CS Peat Peat Duluth Capital WADENA CROW Peat CARLTON OTTER TAIL WING SG Per City WILKIN Peat Peat Peat SG Crushed stone/sand SG 1 and gravel districts MILLE TODD PINE SG LACS MORRISON SG D-G MINERAL SYMBOLS GRANT DOUGLAS KANABEC (Major producing areas) 3 SG BENTON Peat Clay Common clay SG STEARNS TRAVERSE SG SG SG POPE CS Peat ISANTI Crushed stone STEVENS CS BIG D-G SHERBURNE STONE SG CHISAGO D-G Dimension granite ANOKA D-G Peat CS SWIFT WRIGHT D-L Dimension limestone D-G KANDIYOHI WASHINGTON MEEKER SG SG 5 Fe Iron LAC QUI RAM- Peat CS PARLE CHIPPEWA SEY Steel HENNEPIN SG S-o IS Industrial sand Minneapolis IS St. Paul McLEOD CARVER SG CS SG Lime Lime plant YELLOW RENVILLE SG Lime SG DAKOTA MEDICINE Clay CS SG Peat Peat Clay SCOTT CS SIBLEY D-G LE Per Perlite plant Clay Peat LINCOLN LYON REDWOOD SUEUR GOODHUE NICOLLET IS SG CS SG S-o Sulfur (oil) RICE SG WABASHA CS SG D-L BROWN CS 4 Clay CS SG SG SG Construction sand BLUE STEELE DODGE and gravel PIPE- COTTON- CS SG D-L MURRAY EARTH WASECA CS CS STONE WOOD WATONWAN OLMSTED SG WINONA CS Steel Steel plant SG SG CS CS 6 Rochester Concentration of MOWER FILLMORE HOUSTON mineral operations ROCK NOBLES JACKSON MARTIN FARIBAULT FREEBORN CS CS CS SG 0 100 Kilometers Source: Minnesota Department of Natural Resources, Division of Lands and Minerals/U.S. -

Mineral Development in Minnesota
MINNESOTA GEOLOGICAL SURVEY D.L. Southwick, Director MINERAL DEVELOPMENT IN MINNESOTA PAST HISTORY, PRESENT TRENDS, AND FUTURE POSSIBILITIES G.B. MOREY Report of Investigations 52 ISSN 0076-9177 UNIVERSITY OF MINNESOTA NOV 6 2UOD, Saint Paul-1998 DEt,;~",' " MINERAL DEVELOPMENT IN MINNESOTA PAST HISTORY, PRESENT TRENDS, AND FUTURE POSSIBILITIES Minnesota Geological Survey 2642 University Avenue West Saint Paul, Minnesota 55114-1057 Telephone: 612-627-4780 Fax: 612-627-4778 E-mail address:[email protected] Web Site: http://geolab.geo.umn.edu/mgs ©1998 by the Board of Regents of the University of Minnesota All rights reserved. The University of Minnesota is committed to the policy that all persons shall have equal access to its programs, facilities, and employment without regard to race, color, creed, religion, national origin, sex, age, marital status, disability, puclic assistance status, veteran status, or sexual orientation. ii CONTENTS Page ABSTRACT .......................................................................................................................................................................... 1 INTRODUCTION ................................................................................................................................................................. 2 A BRIEF mSTORY OF MINING IN MINNESOTA ........................................................................................................... 2 IMPORTANCE OF MINING IN MINNESOTA ................................................................................................................. -

Magney Snively Natural Area Nomination
DRAFT NOMINATION OF MAGNEY SNIVELY AREA TO THE DULUTH NATURAL AREAS PROGRAM April 2003 Nominated by: City of Duluth in cooperation with The Nature Conservancy Magney Snively DNAP Nomination Page 1 5/8/2003 TABLE OF CONTENTS Nomination in Brief About the Nominators Background Eligibility Ownership Criteria Discussion of Site Characteristics and Issues Science Criteria Significant Native Plant Communities Area Special Species Area Geological Landforms Area Appendices Appendix A: Resource List Appendix B: Qualifications of Individual Nominators Appendix C: Real Estate Status and Legal Information Maps Map 1: Nominated Area Map 2a and 2b: Ownership Map 3: Native Plant Communities Map 4: Land Type Associations Map 5: Geologic Landforms Magney Snively DNAP Nomination Page 2 5/8/2003 NOMINATION IN BRIEF What: A nomination of land, referred to as "Magney Snively," for inclusion into the Duluth Natural Area Program (DNAP) in accordance with the Guidelines as directed by ordinance under Article XXIX of Chapter 2 of the Duluth City Code, 1959, as amended. This area is being nominated under the Significant Native Plant Communities Area, Special Species Area, and Geological Landforms Area as described in the DNAP Guidelines under Article IV, Sections A, B, and E. Who: The City of Duluth is nominating the Magney Snively area with technical support from The Nature Conservancy. Where: The Magney Snively nomination includes approximately 1,800 acres in west Duluth. Almost all of the lands nominated include available parcels owned by the City of Duluth, St. Louis County, and The Nature Conservancy (Map 1, 2a, and 2b). Why: Significant Native Plant Communities - Magney Snively is a highly functional viable ecosystem, an extremely rare occurrence in an urban area. -

Field Trip 2
FIELD TRIP 4 GEOLOGY OF THE NICKEL LAKE MACRODIKE AND ITS ASSOCIATION WITH CU-NI-PGE MINERALIZATION IN THE NORTHERN SOUTH KAWISHIWI INTRUSION, DULUTH COMPLEX, NORTHEASTERN MINNESOTA By Dean M. Peterson1 and Paul B. Albers2 1 University of Minnesota Duluth, Natural Resources Research Institute 2 Duluth Metals Limited, Ely, Minnesota “Imagination is more important than knowledge” Albert Einstein INTRODUCTION Mineral deposits are concentrations of specific ore-minerals that society utilizes in immeasurable ways. They have formed from Archean times up to the present, and vary greatly in commodity, mineralogy, alteration, trace element signature, geophysical properties, and grade-tonnage. Individual ore deposits are always unique, and this uniqueness arises from two main sources: 1) fundamental differences in geologic processes and environments; and 2) local, site-specific, geologic variations and associated bounding geometries. The seemingly limitless number of permutations of these (and other) features of mineral deposits defies imagination. Geologists employed in the search for ore deposits have, over the last century, developed the intellectual concept of Ore Deposit Models, which seek to organize many of these variables, lump individual mineral deposits into classes, and establish criteria to aid in mineral exploration (Peterson, 2001a). Such models may be strictly empirical – a collection of observable facts associated with the occurrence of certain metals in economic proportions – or genetic – which attempts 1 to describe the physical -

Geology and Genesis of the Duluth Complex and Its Ore Deposits
JIM MILLER PRECAMBRIAN RESEARCH CENTER DEPT. OF GEOLOGICAL SCIENCES UNIVERSITY OF MINNESOTA DULUTH Minnesota Ground Water Association Spring Meeting May 6, 2010 Talk Outline • History of Geologic Mapping • Evolution of the Midcontinent Rift •Geology & Genesis of the Duluth Complex •Cu-Ni-PGE Mineralization Stillwater Complex, Montana Skaergaard, East Greenland Sudbury Complex, Second Ontario DuluthDuluth Largest Complex Great Complex Dyke, Gabbro Zimbabwe Complex in Bushveld Complex, the World South Africa Geology of Minnesota circa 1872 Minnesota Geological Survey 1872 - 1900 N.H. Winchell The Grout and F.F. Grout Schwartz Era 1911-1958 G.M. Schwartz Saganaga Lake, Aug. 1922 “Chicken-track” Reconnaissance Mapping Sonju Lake area geology 1939 Grout, 1918 Geology of Northeast Minnesota circa 1932 The Quadrangle Mapping Era 1961 - 1982 P.K. Sims, MGS Director 1961-1973 Matt Walton, MGS Director, 1973-1987 J.C. Green – North Shore Volcanics P.W. Weiblen - Bald Eagle Int, Long Is. Lk. W.C. Phinney - NW Duluth Complex D.M. Davidson Jr. - E Duluth Complex B. Bonnichsen - S Duluth Complex, Babbitt Ph.D. Theses R.B. Taylor – Duluth area H.D. Hathan – Gunflint Trail area E.A. Mathez – Logan Sills M.G. Mudrey – Pigeon Point R.W. Cooper – Babbitt/Harris Lake area M.P. Foose – Harris Lake area MGS Misc. Map M-1 M.S. Theses 1964, R.B. Taylor N.W. Jones – Hovland Db, Logan Sills J.A. Kilburg – Ely’s Peak J.R. Burnell – Brule Lake R.J. Stevenson – Sonju Lake Intrusion N.M. Pope – Silver Cliff/Lafayette Bluff John Green Bill Phinney Paul Weiblen Gooseberry Falls, 1996 Gabbro Lake 15’ Quadrangle MGS Miscellaneous Map M-2 Green, Phinney, & Weiblen, 1966 massive sulfide, basal DC Phinney Nathan/Mathez Bonnichsen Davidson Two Harbors Sheet J.C. -

Zircon Grains Recording Magma Evolution History
Significant Zr isotope variations in single zircon grains recording magma evolution history Jing-Liang Guoa,b,1, Zaicong Wanga,1, Wen Zhanga, Frédéric Moyniera,c, Dandan Cuia, Zhaochu Hua, and Mihai N. Duceab,d aState Key Laboratory of Geological Processes and Mineral Resources, School of Earth Sciences, China University of Geosciences, Wuhan 430074, China; bDepartment of Geosciences, University of Arizona, Tucson, AZ 85718; cInstitut de Physique du Globe de Paris, Université de Paris, CNRS, 75238 Paris Cedex 05, France; and dFaculty of Geology and Geophysics, University of Bucharest, 010041 Bucharest, Romania Edited by Bruce Watson, Rensselaer Polytechnic Institute, Troy, NY, and approved July 20, 2020 (received for review February 27, 2020) Zircons widely occur in magmatic rocks and often display internal differentiation processes of the silicate Earth (17, 18), the Zr zonation finely recording the magmatic history. Here, we presented isotopes of zircon might also record related messages. in situ high-precision (2SD <0.15‰ for δ94Zr) and high–spatial-resolution Recent developments of bulk and in situ analytical methods (20 μm) stable Zr isotope compositions of magmatic zircons in a have permitted studies on mass-dependent Zr isotope fraction- suite of calc-alkaline plutonic rocks from the juvenile part of the ation in terrestrial samples, including zircons (15, 19–24). They Gangdese arc, southern Tibet. These zircon grains are internally show the great potential of Zr isotope in tracing magmatic zoned with Zr isotopically light cores and increasingly heavier processes. However, available data have been rather limited and rims. Our data suggest the preferential incorporation of lighter resulted in very controversial interpretation (21, 22), such as for Zr isotopes in zircon from the melt, which would drive the residual fractionation factor α (whether greater or smaller than 1). -
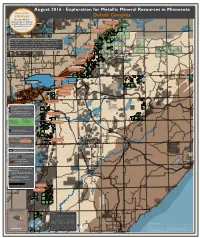
Exploration for Metallic Mineral Resources in Minnesota DULUTH COMPLEX Duluth Complex
August 2016 - Exploration for Metallic Mineral Resources: Copper, Nickel, and Platinum Group Metals, Duluth Complex Map The August 2016 - Exploration for Metallic Mineral Resources in Minnesota DULUTH COMPLEX Duluth Complex Hosting World T. 62, R. 11 BWCAW White B2 D3 Gull Spruce Road Deposit Cu,Ni,PGM Class Copper, Nickel, Iron Gabbro Lake Twin Metals Minnesota NI 43-101 Lake and Platinum Group T. 62, R. 12 Lake Nickel Lake A B2 B2 T. 62, R. 7 T. 62, R. 10 T. 62, R. 9 Quadga Isabella S. Filson Creek Deposit Cu,Ni,PGM B2 E3 T. 62, R. 8 Metal Resources 1 D3 Lake Lake T. 62, R. 13 Encampment Minerals E3 E T3 D3 Omaday Bald Eagle Johnson B2 T3 Exploring since 1948 Purvis Lake-Ober One Pine E Lake Lake T. 62, R. 14 Lake Maturi Deposit Cu,Ni,PGM T3 E D3 Foundation SNA Lake B2 T3 T T3 T E D3 Twin Metals Minnesota NI 43-101 B2 T3 E E Bogberry T To w e r T E E3 Lake Bear B2 T D3 BWCAW T B2 B2 D3 Bog Head B2 T3 E3 Lake Lake T Bear Island T3 T B2 E3 E3 D3 T. 61, R. 14 R. 61, T. State Boundary Waters Canoe Area Wilderness (BWCAW) Bear F Park Lake T3B2 T Head F F D3 E3 E D3 There are many deposits of copper, nickel, and PlatinumLake Group Birch E L E Harris D3 T. 61, R. 12 B2 Lake E Metal (PGM) resources in the bedrock along approximately 40 miles L Lake T. -

Relationship of Structural Geology of the Duluth Complex to Economic Mineralization
MINNESOTA DEPARTMENT OF NATURAL RESOURCES Division of Minerals Report 241-2 RELATIONSHIP OF STRUCTURAL GEOLOGY OF THE DULUTH COMPLEX TO ECONOMIC MINERALIZATION A Final Report to the Minnesota Department of Natural Resources and the Natural Resources Research Institute of the University of Minnesota Duluth by Timothy B. Holst, Eugene E. Mullenmeister, Val W. Chandler, John C. Green, and Paul w. Weiblen Funded by The Legislative Commission on Minnesota Resources The Natural Resources Research Institute TABLE OF CONTENTS I. Introduction I-1 II. Structural Analysis of the Northeast Mesabi Range II-1 III. Structural Analysis of the Dunka River Area III-1 IV. The Form of the Base of the Duluth Complex IV-1 v. Field Mapping V-1 VI. Geophysical Studies VI-1 VII. Discussion, Interpretation, Conclusions VII-1 VIII. Further Work VIII-1 References R-1 Maps: ~)Ire·,:,,,( Plate I: ~Geology of the Northeast Mesabi Range Plate II: Structure Contour Map on the Upper Contact of the Lower Slatey Member of the Biwabik Iron Formation in the Dunka Pit Area Plate III: Structure Contour Map of the Base of the Duluth Complex in the Minnamax Area Plate IV: Structure Contour Map on the Base of the Duluth Complex From the Dunka River Area to Hoyt Lakes Plate V: Structure Contour Map on the Base of the Duluth Complex in the Dunka River Area Plate VI: Structural Geology of the Dunka River Area Cross Sections: A: Minnamax Project Area B: Minnamax Project Area C: Minnamax Project Area D: Dunka River Area INTRODUCTION A series of mafic igneous rocks (both intrusive and extrusive), known from outcrop or inferred from geophysical data, forms an arc that runs from Kansas, through Iowa and Minnesota, through Lake Superior, and south through Michigan, perhaps into Ohio. -

Palaios 2010 Book Review PALAIOS DOI: 10.2110/Palo.2010.BR59 Emphasizing the Impact of Life on Earth’S History
PALAIOS 2010 Book Review PALAIOS DOI: 10.2110/palo.2010.BR59 Emphasizing the impact of life on Earth’s history Roadside Geology of Minnesota, edited by Richard W. Ojakangas, 2009, Mountain Press Publishing Company, Missoula, Montana, 353 p., USD 26.00, paper, ISBN: 978087425624. Having received part of my geology education in Minnesota, explanations for the various road stops highlights the develop- the opportunity to review Roadside Geology of Minnesota by ment of a late Archean greenstone belt composed of volcanic and Richard W. Ojakangas was a pleasure. The guidebook brought related sedimentary rocks metamorphosed and deformed during back fond memories of field trips to some of the sites covered the Algoman orogenic event about 2.7 billion years ago. One in the book. Dr. Ojakangas is a native Minnesotan and profes- stop in particular shows well-preserved pillowed basalts within sor emeritus in the Department of Geological Sciences at the the greenstone sequence. Also highlighted in this section is the University of Minnesota Duluth. He has written the book in an Mesabi Iron Range, known for producing high-grade iron ore easy-to-follow manner that targets the novice interested in learn- from the early Proterozoic Biwabik Iron Formation. The book ing about Minnesota geologic history. Geologists and geology includes stops to view the iron formation in road cuts along U.S. students will also find the book valuable as reference tool. 53 near the town of Eveleth and a stop to view mining operations The book covers over 3.5 billion years of Earth’s history, at an overlook near the city of Virginia. -

Geologic Map of Minnesota Bedrock Geology
STATE MAP SERIES S-21 Bedrock Geology GEOLOGIC MAP OF MINNESOTA BEDROCK GEOLOGY Compiled by Mark A. Jirsa, Terrence J. Boerboom, V.W. Chandler, John H. Mossler, Anthony C. Runkel, and Dale R. Setterholm 2011 INTRODUCTION This map is a new construct that incorporates existing geologic maps where prior mappers had adequate ground control, and new interpretations based on drill hole, geophysical, and unpublished data where they did not. The interpretation differs significantly from previous maps to reflect new data and accommodate scale. It portrays our current geologic understanding of the temporal and geographic distribution of units within major Precambrian terranes (Fig. 1), and of the Phanerozoic strata. The western part of the map is inferred largely from geophysical maps, anchored locally by drilling. In many places, contacts are drawn between units of the same or similar apparent rock type (and same unit label); these are recognized as geometrically distinct, though geophysically or lithologically similar. Digital files corresponding to this map allow removal of Cretaceous, Paleozoic, and some parts of Mesoproterozoic strata to reveal an interpretation of the underlying Precambrian bedrock. DESCRIPTION OF MAP UNITS PHANEROZOIC ROCKS MESOZOIC Cretaceous Ku Undifferentiated—Conglomerate, sandstone, mudstone, shale, marlstone, siltstone, and minor lignite, deposited in marine and non-marine settings; likely Cenomanian to Campanian age. Unit outline is the product of contouring the stratigraphic top and base, from which an isopach grid was created. Because the distribution is patchy, unit boundaries were drawn from the gridded data to represent locations where more than 25 feet (8 meters) of thickness occurs. As a result, many areas outside of the unit boundaries may be overlain by thin Cretaceous strata and the unit is depicted without a contact line.