Overview of the Lady Beetle Tribe Diomini (Coleoptera: Coccinellidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ladybirds, Ladybird Beetles, Lady Beetles, Ladybugs of Florida, Coleoptera: Coccinellidae1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. EENY-170 Ladybirds, Ladybird beetles, Lady Beetles, Ladybugs of Florida, Coleoptera: Coccinellidae1 J. H. Frank R. F. Mizell, III2 Introduction Ladybird is a name that has been used in England for more than 600 years for the European beetle Coccinella septempunctata. As knowledge about insects increased, the name became extended to all its relatives, members of the beetle family Coccinellidae. Of course these insects are not birds, but butterflies are not flies, nor are dragonflies, stoneflies, mayflies, and fireflies, which all are true common names in folklore, not invented names. The lady for whom they were named was "the Virgin Mary," and common names in other European languages have the same association (the German name Marienkafer translates Figure 1. Adult Coccinella septempunctata Linnaeus, the to "Marybeetle" or ladybeetle). Prose and poetry sevenspotted lady beetle. Credits: James Castner, University of Florida mention ladybird, perhaps the most familiar in English being the children's rhyme: Now, the word ladybird applies to a whole Ladybird, ladybird, fly away home, family of beetles, Coccinellidae or ladybirds, not just Your house is on fire, your children all gone... Coccinella septempunctata. We can but hope that newspaper writers will desist from generalizing them In the USA, the name ladybird was popularly all as "the ladybird" and thus deluding the public into americanized to ladybug, although these insects are believing that there is only one species. There are beetles (Coleoptera), not bugs (Hemiptera). many species of ladybirds, just as there are of birds, and the word "variety" (frequently use by newspaper 1. -

Large Positive Ecological Changes of Small Urban Greening Actions Luis Mata, Amy K. Hahs, Estibaliz Palma, Anna Backstrom, Tyler King, Ashley R
Large positive ecological changes of small urban greening actions Luis Mata, Amy K. Hahs, Estibaliz Palma, Anna Backstrom, Tyler King, Ashley R. Olson, Christina Renowden, Tessa R. Smith and Blythe Vogel WebPanel 2 Table S2.1. List of the 94 insect species that were recorded during the study. DET: Detritivore; HER: Herbivore; PRE: Predator; PAR: Parasitoid. All species are indigenous to the study area, excepting those marked with an *. Year 0 Year 1 Year 2 Year 3 Species/morphospecies Common name Family DET HER PRE PAR [2016] [2017] [2018] [2019] Hymenoptera | Apocrita Apocrita 5 Apocrita 5 Apocrita 33 Apocrita 33 Apocrita 36 Apocrita 36 Apocrita 37 Apocrita 37 Apocrita 40 Apocrita 40 Apocrita 44 Apocrita 44 Apocrita 46 Apocrita 46 Apocrita 48 Apocrita 48 Apocrita 49 Apocrita 49 Apocrita 51 Apocrita 51 Apocrita 52 Apocrita 52 Apocrita 53 Apocrita 53 Apocrita 54 Apocrita 54 Apocrita 55 Apocrita 55 Apocrita 56 Apocrita 56 Apocrita 57 Apocrita 57 Apocrita 58 Apocrita 58 Apocrita 59 Apocrita 59 Apocrita 60 Apocrita 60 Apocrita 61 Apocrita 61 Apocrita 62 Apocrita 62 Apocrita 65 Apocrita 65 Apocrita 74 Apocrita 74 Apocrita 89 Apocrita 89 Apocrita 90 Apocrita 90 Apocrita 91 Apocrita 91 Hymenoptera | Apoidea | Anthophila Anthophila 1 Anthophila 1 Anthophila 3 Anthophila 3 Apis mellifera* European honeybee Apidae Diptera | Brachycera Brachycera 2 Brachycera 2 Brachycera 7 Brachycera 7 Brachycera 8 Brachycera 8 Brachycera 14 Brachycera 14 Brachycera 15 Brachycera 15 Brachycera 16 Brachycera 16 Brachycera 18 Brachycera 18 Brachycera 19 Brachycera -

Section IV – Guideline for the Texas Priority Species List
Section IV – Guideline for the Texas Priority Species List Associated Tables The Texas Priority Species List……………..733 Introduction For many years the management and conservation of wildlife species has focused on the individual animal or population of interest. Many times, directing research and conservation plans toward individual species also benefits incidental species; sometimes entire ecosystems. Unfortunately, there are times when highly focused research and conservation of particular species can also harm peripheral species and their habitats. Management that is focused on entire habitats or communities would decrease the possibility of harming those incidental species or their habitats. A holistic management approach would potentially allow species within a community to take care of themselves (Savory 1988); however, the study of particular species of concern is still necessary due to the smaller scale at which individuals are studied. Until we understand all of the parts that make up the whole can we then focus more on the habitat management approach to conservation. Species Conservation In terms of species diversity, Texas is considered the second most diverse state in the Union. Texas has the highest number of bird and reptile taxon and is second in number of plants and mammals in the United States (NatureServe 2002). There have been over 600 species of bird that have been identified within the borders of Texas and 184 known species of mammal, including marine species that inhabit Texas’ coastal waters (Schmidly 2004). It is estimated that approximately 29,000 species of insect in Texas take up residence in every conceivable habitat, including rocky outcroppings, pitcher plant bogs, and on individual species of plants (Riley in publication). -

Surveying for Terrestrial Arthropods (Insects and Relatives) Occurring Within the Kahului Airport Environs, Maui, Hawai‘I: Synthesis Report
Surveying for Terrestrial Arthropods (Insects and Relatives) Occurring within the Kahului Airport Environs, Maui, Hawai‘i: Synthesis Report Prepared by Francis G. Howarth, David J. Preston, and Richard Pyle Honolulu, Hawaii January 2012 Surveying for Terrestrial Arthropods (Insects and Relatives) Occurring within the Kahului Airport Environs, Maui, Hawai‘i: Synthesis Report Francis G. Howarth, David J. Preston, and Richard Pyle Hawaii Biological Survey Bishop Museum Honolulu, Hawai‘i 96817 USA Prepared for EKNA Services Inc. 615 Pi‘ikoi Street, Suite 300 Honolulu, Hawai‘i 96814 and State of Hawaii, Department of Transportation, Airports Division Bishop Museum Technical Report 58 Honolulu, Hawaii January 2012 Bishop Museum Press 1525 Bernice Street Honolulu, Hawai‘i Copyright 2012 Bishop Museum All Rights Reserved Printed in the United States of America ISSN 1085-455X Contribution No. 2012 001 to the Hawaii Biological Survey COVER Adult male Hawaiian long-horned wood-borer, Plagithmysus kahului, on its host plant Chenopodium oahuense. This species is endemic to lowland Maui and was discovered during the arthropod surveys. Photograph by Forest and Kim Starr, Makawao, Maui. Used with permission. Hawaii Biological Report on Monitoring Arthropods within Kahului Airport Environs, Synthesis TABLE OF CONTENTS Table of Contents …………….......................................................……………...........……………..…..….i. Executive Summary …….....................................................…………………...........……………..…..….1 Introduction ..................................................................………………………...........……………..…..….4 -
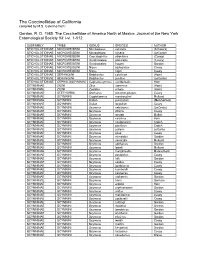
The Coccinellidae of California Compiled by M.S
The Coccinellidae of California compiled by M.S. Caterino from: Gordon, R. D. 1985. The Coccinellidae of America North of Mexico. Journal of the New York Entomological Society 93: i-vi, 1-912. SUBFAMILY TRIBE GENUS SPECIES AUTHOR STICHOLOTIDINAE MICROWEISEINI Microweisea suturalis (Schwarz) STICHOLOTIDINAE MICROWEISEINI Microweisea misella (LeConte) STICHOLOTIDINAE MICROWEISEINI Coccidophilus atronitens (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea planiceps (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea hageni Gordon STICHOLOTIDINAE MICROWEISEINI Nipus biplagiatus Casey STICHOLOTIDINAE MICROWEISEINI Nipus niger Casey STICHOLOTIDINAE SERANGIINI Delphastus catalinae (Horn) STICHOLOTIDINAE SERANGIINI Delphastus pusillus (LeConte) STICHOLOTIDINAE CEPHALOSCYMNINI Cephaloscymnus occidentalis Horn SCYMNINAE ZILINI Zilus aterrimus (Horn) SCYMNINAE ZILINI Zagloba ornata (Horn) SCYMNINAE STETHORINI Stethorus punctum picipes Casey SCYMNINAE SCYMNINI Cryptolaemus montrouzieri Mulsant SCYMNINAE SCYMNINI Didion punctatum (Melsheimer) SCYMNINAE SCYMNINI Didion longulum Casey SCYMNINAE SCYMNINI Scymnus nebulosus (LeConte) SCYMNINAE SCYMNINI Scymnus dificilis Casey SCYMNINAE SCYMNINI Scymnus fenderi Malkin SCYMNINAE SCYMNINI Scymnus caurinus Horn SCYMNINAE SCYMNINI Scymnus coniferarum Crotch SCYMNINAE SCYMNINI Scymnus pacificus Crotch SCYMNINAE SCYMNINI Scymnus pallens LeConte SCYMNINAE SCYMNINI Scymnus gilae Casey SCYMNINAE SCYMNINI Scymnus mimoides Gordon SCYMNINAE SCYMNINI Scymnus cervicalis Mulsant SCYMNINAE SCYMNINI Scymnus apithanus Gordon -

Forest Health Technology Enterprise Team
Forest Health Technology Enterprise Team TECHNOLOGY TRANSFER Biological Control ASSESSING HOST RANGES FOR PARASITOIDS AND PREDATORS USED FOR CLASSICAL BIOLOGICAL CONTROL: A GUIDE TO BEST PRACTICE R. G. Van Driesche and R. Reardon, Editors Forest Health Technology Enterprise Team—Morgantown, West Virginia United States Forest FHTET-2004-03 Department of Service September 2004 Agriculture __________________________________ ASSESSING HOST RANGES OF PARASITOIDS AND PREDATORS CHAPTER 1. INTRODUCTION PREDICTING HOST RANGES OF PARASITOIDS AND PREDACIOUS INSECTS—WHAT ARE THE ISSUES? R. G. Van Driesche Department of Plant, Soil and Insect Science: Division of Entomology, University of Massachusetts, Amherst, MA 01003 USA [email protected] GOALS FOR HOST RANGE TESTING Estimating the likely nontarget impacts of agents released to suppress invasive plants has been legally required, to one degree or another, for many decades. Similar predictions were not formally required for introductions of parasitoids or predators of pest arthropods. That is now beginning to change. This book has as its goal an exploration of how such estimates can best be made. This requires overcoming a series of problems, some logistical, some technical, some tied to an unclear theoretical framework for the activity. In this book, the editors and authors have tried to address many of these needs, in some chapters as essays on important tasks that need to be achieved, in other chapters as case history explorations of how the tasks were done in particular cases. This book will not be the final answer, but we hope it might propel the search for such an answer along. LEGAL REQUIREMENTS Whether or not predicting the host ranges of parasitoids and predators is legally required varies among countries. -

Four New Ladybug Species Belonging to Decadiomus Chapin (Coleoptera: Coccinellidae) from Puerto Rico
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/284835268 Four New Ladybug Species Belonging to Decadiomus Chapin (Coleoptera: Coccinellidae) from Puerto Rico Article in Neotropical Entomology · December 2014 DOI: 10.1007/s13744-014-0243-8 CITATIONS READS 2 226 2 authors: Alejandro E. Segarra Miriel Otero University of Puerto Rico at Mayagüez Montana State University 39 PUBLICATIONS 208 CITATIONS 2 PUBLICATIONS 8 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Establishment of the Sea Grape Flatid, Petrusa epilepsis, in Florida. View project A NEW SPECIES OF ABBROSOGA (HEMIPTERA: FULGOROIDEA: DELPHACIDAE), AN ENDEMIC PUERTO RICAN GENUS View project All content following this page was uploaded by Miriel Otero on 23 October 2017. The user has requested enhancement of the downloaded file. Neotrop Entomol (2014) 43:555–563 DOI 10.1007/s13744-014-0243-8 SYSTEMATICS, MORPHOLOGY AND PHYSIOLOGY Four New Ladybug Species Belonging to Decadiomus Chapin (Coleoptera: Coccinellidae) from Puerto Rico 2 1 AE SEGARRA-CARMONA ,MOTERO 1College of Agricultural Sciences, Agricultural Experiment Station, Dept of Crops & Agro-Environmental Sciences, Univ of Puerto Rico, Mayagüez, PR, USA 2Dept of Crops & Agro-Environmental Sciences, Univ of Puerto Rico, Mayagüez, PR, USA Keywords Abstract Caribbean, Coccinellidae, Decadiomus, While searching for native natural enemies attacking invasive insect pests new species, Puerto Rico in Puerto Rico, we found four undescribed ladybug species belonging to Correspondence the Caribbean ladybug genus Decadiomus Chapin. In this article, we AE Segarra-Carmona, Dept of Crops & Agro- describe the following species from Puerto Rico: Decadiomus seini n. -

Melanaphis Sacchari (Homoptera: Aphididae), a Sugarcane Pest New to Louisiana
Scientific Notes 435 MELANAPHIS SACCHARI (HOMOPTERA: APHIDIDAE), A SUGARCANE PEST NEW TO LOUISIANA W. H. WHITE1, T. E. REAGAN2 AND D. G. HALL3 1USDA, ARS, Sugarcane Research Unit, P. O. Box 470, Houma, LA 70361-0470 2Department of Entomology, 402 Life Sciences Bldg., Louisiana State University, Baton Rouge, LA 70803 3Research Department, United States Sugar Corp., P. O. Drawer 1207, Clewiston, FL 33440 While inspecting sugarcane (interspecific hy- can be associated with other sugarcane homopter- brids of Saccharum spp.) varietal trials on the ans (principally the West Indian canefly, Saccha- USDA-ARS Ardoyne Research Farm near Houma, rosydne saccharivora Westwood), sooty mold alone LA on 9 September 1999, we noticed an infesta- could not be used as a means of verifying the oc- tion of an aphid unfamiliar in Louisiana sugar- currence of the aphid in our sample fields. cane. Specimens were collected and sent to the Sugarcane aphids were found in eight of the 21 USDA-ARS Systematic Entomology Laboratory, parishes surveyed (38%) (Fig. 1). We did not find Beltsville, MD for identification. Gary L. Miller aphids at all four sample sites in any given par- identified the specimens as the sugarcane aphid, ish. Generally, where sugarcane aphids were de- Melanaphis sacchari (Zehntner). The collection of tected their infestation densities were low. this species represents a new distribution record Overall, the survey indicated the aphid has al- for the continental United States and holdings of ready spread throughout much of the sugarcane the National Aphidoidea Collection, which until growing areas in Louisiana. The low population now included specimens from Florida and Hawaii. -

1 the RESTRUCTURING of ARTHROPOD TROPHIC RELATIONSHIPS in RESPONSE to PLANT INVASION by Adam B. Mitchell a Dissertation Submitt
THE RESTRUCTURING OF ARTHROPOD TROPHIC RELATIONSHIPS IN RESPONSE TO PLANT INVASION by Adam B. Mitchell 1 A dissertation submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Entomology and Wildlife Ecology Winter 2019 © Adam B. Mitchell All Rights Reserved THE RESTRUCTURING OF ARTHROPOD TROPHIC RELATIONSHIPS IN RESPONSE TO PLANT INVASION by Adam B. Mitchell Approved: ______________________________________________________ Jacob L. Bowman, Ph.D. Chair of the Department of Entomology and Wildlife Ecology Approved: ______________________________________________________ Mark W. Rieger, Ph.D. Dean of the College of Agriculture and Natural Resources Approved: ______________________________________________________ Douglas J. Doren, Ph.D. Interim Vice Provost for Graduate and Professional Education I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. Signed: ______________________________________________________ Douglas W. Tallamy, Ph.D. Professor in charge of dissertation I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. Signed: ______________________________________________________ Charles R. Bartlett, Ph.D. Member of dissertation committee I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. Signed: ______________________________________________________ Jeffery J. Buler, Ph.D. Member of dissertation committee I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. -

New State and Island Records of Coccinellidae (Coleoptera) in Hawai‘I, USA
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 9-25-2020 New state and island records of Coccinellidae (Coleoptera) in Hawai‘i, USA Louis S. Hesler William D. Perreira Dana Anne Yee Joshua H.S. Silva Follow this and additional works at: https://digitalcommons.unl.edu/insectamundi Part of the Ecology and Evolutionary Biology Commons, and the Entomology Commons This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. A journal of world insect systematics INSECTA MUNDI 0795 New state and island records of Coccinellidae (Coleoptera) Page Count: 4 in Hawai‘i, USA Louis S. Hesler USDA Agricultural Research Service, 2923 Medary Avenue, Brookings, SD 57006, USA [email protected] William D. Perreira P.O. Box 61547, Honolulu, HI 96839, USA Dana Anne Yee Dana Anne Yee Foundation, 1717 Mott Smith Drive #905, Honolulu, HI 96822, USA Joshua H.S. Silva University of Hawai‘i Cooperative Extension, 955 Kamehameha Highway, University of Hawai‘i at Manoa, Pearl City, HI 96782, USA Date of issue: September 25, 2020 Center for Systematic Entomology, Inc., Gainesville, FL Hesler LS, Perreira WD, Yee DA, Silva JHS. 2020. New state and island records of Coccinellidae (Coleoptera) in Hawai‘i, USA. Insecta Mundi 0795: 1–4. Published on September 25, 2020 by Center for Systematic Entomology, Inc. P.O. Box 141874 Gainesville, FL 32614-1874 USA http://centerforsystematicentomology.org/ Insecta Mundi is a journal primarily devoted to insect systematics, but articles can be published on any non- marine arthropod. -

Heidi Liere Department Environmental Studies Seattle University 910 12Th Ave, Seattle WA [email protected]; (206) 296-4035
Heidi Liere Curriculum vitae Page 1 of 9 Heidi Liere Department Environmental Studies Seattle University 910 12th Ave, Seattle WA [email protected]; (206) 296-4035 Research interests urban and rural agroecology, insect community ecology, sustainable food systems, tropical biology, conservation biology, natural pest control Professional appointments 2018-present Assistant Professor, Environmental Studies, Seattle University 2014- 2018 Visiting Scholar, Biology Department, Reed College, Portland 2013- 2014 Visiting Assistant Professor, Biology Department, The University of the South- Sewanee, Tennessee 2011- 2012 Postdoctoral Research Associate, Department of Entomology, University of Wisconsin-Madison and the Great Lakes Bioenergy Research Center Education • Ph.D. Ecology and Evolutionary Biology (EEB), University of Michigan, USA, 2005-2011 • M.Sc. Ecology and Evolutionary Biology, University of Michigan, USA, 2004-2005 • Licenciatura in Biology (B.S. equivalent)--Cum laude-- Universidad del Valle de Guatemala, Guatemala City, 1996-2001 Research grants and fellowships Current 2021-2023: “Effect of the abundance, quality, and connectivity of green-spaces on beneficial insects in urban community gardens”. The Murdock College Research Program for Natural Sciences ($65,000) 2020-2023: “Ecological Networks, Management Shifts and Ecosystem Services in Urban Agricultural Landscapes.” USDA-NIFA grant. Co-PI. In collaboration with Stacy Philpott (University of California-Santa Cruz), Shalane Jha (University of Texas- Austin) and Brenda Lin (CISRO-Australia) ($500,000) 2020-2021: Global Engagement Grant, Seattle University. Collaborative pilot project: biodiversity inhome vegetable gardens in Guatemala ($6,320) 2019-2020: College of Arts and Sciences Student assistant award, Seattle University ($800) Heidi Liere Curriculum vitae Page 2 of 9 Past 2019: Undergraduate Student/Faculty Research Support Award, Hoba Endowment, College of Science and Engineering, Seattle University ($3250) 2019: Summer Faculty Fellowship Program, Seattle University. -

Coccinellids Associated with the Cotton Aphid (Homoptera: Aphididae) in Northeast Arkansas Cotton'
Coccinellids Associated with the Cotton Aphid (Homoptera: Aphididae) in Northeast Arkansas Cotton' Hugh E. Conway 2 and Timothy J. Kring3 USDA-APHIS-PPQ-CPHST-PDDML Pest Detection, Diagnostic & Management Laboratory, Edinburg, Texas 78541 USA J. Entomol. Sci. 45(2): 129-139 (April 2010) Abstract Adult and larval coccinellids are important predators of the cotton aphid, Aphis gos- sypii Glover, in cotton, Gossypium hirsutum L. Adult and larval Coccinellinae and Scymninae were sampled by beat-pan method and identified to species in a 3-year field study (1999-2001) conducted in northeast Arkansas. The coccinellids observed in descending order of abundance were Hippodarnia convergens Guerin, Scymninae (Scymnus spp. and Diomus spp.), Coleome- gil/a macu/ata (Degeer), Harmonia axyridis (Pallas), and Coccinella septempunctata L. Popula- tion dynamics and community structure by species for C. maculata, H. axyridis and C. septempunctata were unpredictable within and among years. Based on population densities, timing of colonization, and consistent delayed density-dependent relationship with aphid popula- tions, H. convergens and Scymninae genera (Scymnus spp. and Diomus spp.) were the most important predators of cotton aphids in northeast Arkansas. Key Words coccinellid, Coccinellinae, Scymninae, cotton aphid, Aphis gossypii The diverse beneficial insect fauna found in cotton, Gossypium hirsutum L., is well described (Whitcomb and Bell 1964, van den Bosch and Hagen 1966, Wells et al. 2001). The natural enemies commonly credited with controlling the cotton aphid, Aphis gossypll Glover, are the predaceous arthropods including the coccinellids, chrysopids, and syrphids, the parasitoids (braconids), and the entomogenous fungus, Neozygites fresenll (Nowakowski) Batko (Chambers 1986, Frazer 1988, Rosenheim and Cisneros 1994, Steinkraus et al.