Solubility of Stevioside and Rebaudioside a in Water, Ethanol and Their Binary Mixtures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FEMA GRAS 29 December 2019 SUPPLEMENTARY INFORMATION 1
SUPPLEMENTARY INFORMATION 1: Identity for Natural Flavor Complexes as Evaluated by the Expert Panel The Identification Description as Reviewed by the FEMA FEMA No.1 FEMA Primary Name Expert Panel Rebaudioside M ≥80%; Rebaudioside D 5-20%; Total 4895 Rebaudioside M steviol glycosides ≥95%. Glutamic acid 35-40%; Other amino acids 1-2%; Total Corynebacterium glutamicum corn nitrogen 6-7%; Aliphatic primary alcohols, aldehydes, 4907 syrup fermentation product carboxylic acids, acetals and esters containing additional oxygenated functional groups 1-2%; Minerals 9-11% Inosine 5´-monophosphate 20-25%; Amino acids 7-8%; Corynebacterium stationis corn 4908 Minerals 23-25%; water 28-37%; Other nucleotides 1-2%; syrup fermentation product Total nitrogen 5-8% Supraglucosylated steviol glycosides 70-80%; Rebaudioside Glucosylated steviol glycosides, 4909 A 14-20%; Steviol glycosides not further glucosylated, each 70-80% individually, not to exceed 3%; Maltodextrin 3-10% Supraglucosylated steviol glycosides 30-40%; Rebaudioside Glucosylated steviol glycosides, A 5-8%; Not more than 4% stevioside; All other individual 4910 40% steviol glycosides not further glucosylated <3%; Maltodextrin 45-60% Stevioside 70-80%; Rebaudioside A 13-18%; Steviobioside 1- 3%; Rebaudioside C 2-3%; Total glycosides (including 4911 Stevia extract stevioside, 70% Rebaudioside D, Rebaudioside B, Rebaudioside F, Dulcoside A, and Rubusoside) <3% Derived from hibiscus blossom calyces (Hibiscus sabdariffa L.) , Hibiscus blossom extract is measured as water 30-60%; 4912 Hibiscus -

Beneficial Effects of Stevioside on Ages, Blood Glucose, Lipid Profile and Renal Status in Streptozotocin-Induced Diabetic Rats
J Appl Biomed journal homepage: http://jab.zsf.jcu.cz DOI: 10.32725/jab.2019.013 Journal of Applied Biomedicine Original research article Beneficial effects of Stevioside on AGEs, blood glucose, lipid profile and renal status in streptozotocin-induced diabetic rats Urmila Aswar 1 *, Vinayak Gogawale 2, Pankaj Miniyar 2, Yugendra Patil 3 1 Bharati Vidyapeeth (Deemed to be University), Poona College of Pharmacy, Erandwane, Pune, Maharashtra, India 2 STES’s Sinhgad Institute of Pharmacy, Savitribai Phule Pune University, Narhe, Pune, Maharashtra, India 3 National Chemical Laboratory, Pune, Maharashtra, India Abstract The advanced glycated end products (AGEs) are formed in the diabetic patients; it is a major cause of macrovascular and microvascular complications in diabetes. Clinically there is no treatment available for the AGEs. Stveoside (Stv), a sweetener has potent anti-diabetic and anti-oxidant activity. Hence, we investigated its use in prevention of AGEs formation using in vitro and in vivo models. Diabetes was induced by streptozotocin (STZ). These rats were kept without treatment till blood HbA1c was markedly increased. They were then divided into 5 groups and treated orally with vehicle or Metformin (MET) or Stv respectively for 28 days. Every 7th day, animals were tested for body weight and blood glucose (BG). On the last day of treatment, all the groups were evaluated for physiological and biochemical parameters, histopathology and AGEs; N-carboxymethyl-lysine (CML) estimation. Stv showed inhibition of AGEs in in vitro as well as in in vivo respectively. Positive effects were seen on the BG, lipid profile and urine parameters as well it showed reduced formation of CML. -

FEMA GRAS 29 December 2019 SUPPLEMENTARY INFORMATION 1
SUPPLEMENTARY INFORMATION 1: Identity for Natural Flavor Complexes as Evaluated by the Expert Panel The Identification Description as Reviewed by the FEMA FEMA No.1 FEMA Primary Name Expert Panel Rebaudioside M ≥80%; Rebaudioside D 5-20%; Total 4895 Rebaudioside M steviol glycosides ≥95%. Glutamic acid 35-40%; Other amino acids 1-2%; Total Corynebacterium glutamicum corn nitrogen 6-7%; Aliphatic primary alcohols, aldehydes, 4907 syrup fermentation product carboxylic acids, acetals and esters containing additional oxygenated functional groups 1-2%; Minerals 9-11% Inosine 5´-monophosphate 20-25%; Amino acids 7-8%; Corynebacterium stationis corn 4908 Minerals 23-25%; water 28-37%; Other nucleotides 1-2%; syrup fermentation product Total nitrogen 5-8% Supraglucosylated steviol glycosides 70-80%; Rebaudioside Glucosylated steviol glycosides, 4909 A 14-20%; Steviol glycosides not further glucosylated, each 70-80% individually, not to exceed 3%; Maltodextrin 3-10% Supraglucosylated steviol glycosides 30-40%; Rebaudioside Glucosylated steviol glycosides, A 5-8%; Not more than 4% stevioside; All other individual 4910 40% steviol glycosides not further glucosylated <3%; Maltodextrin 45-60% Stevioside 70-80%; Rebaudioside A 13-18%; Steviobioside 1- 3%; Rebaudioside C 2-3%; Total glycosides (including 4911 Stevia extract stevioside, 70% Rebaudioside D, Rebaudioside B, Rebaudioside F, Dulcoside A, and Rubusoside) <3% Derived from hibiscus blossom calyces (Hibiscus sabdariffa L.) , Hibiscus blossom extract is measured as water 30-60%; 4912 Hibiscus -

Nutritional and Medicinal Properties of Stevia Rebaudiana
Review Article Curr Res Diabetes Obes J Volume 13 Issue 4 - July 2020 Copyright © All rights are reserved by Fasiha Ahsan DOI: 10.19080/CRDOJ.2020.13.555867 Nutritional and Medicinal Properties of Stevia Rebaudiana Fasiha Ahsan*, Shahid Bashir and Faiz-ul-Hassan Shah University Institute of Diet and Nutritional Sciences, The University of Lahore, Pakistan Submission: June 25, 2020; Published: July 16, 2020 *Corresponding author: Fasiha Ahsan, PhD Scholar, University Institute of Diet and Nutritional Sciences, The University of Lahore, Pakistan Abstract Researches on new molecules with the least toxic effects and better potency is on its way and more attention is being given upon medicinal plants for forcing away the above problems. Medicinal plants have been recognized as potential drug candidates. Stevia, a natural sweetener with medicinal properties and also having nutritional, therapeutic and industrial importance is being used all over the world. Stevia rebaudiana leaves are usually referred to as candy, sweet and honey leaves. Diterpene glycosides are responsible for its high sweetening potential of leaves. The phytochemical properties of bioactive chemicals present in stevia leaves are involves in maintaining the physiological functions of human body. Paper also highlights the importance of nutritional aspects of dried stevia leaves, metabolism of stevia, effects of it consumption on human health and clinical studies related to stevia ingestion. Various medicinal properties of stevia leaves discussed in paper like anti-hyperglycemia, anti-oxidative, hypotensive, nephro-protective, hepato protective, antibacterial and antifungal. Basic purpose of this review to understand the medicinalKeywords: potential Stevia; Diabetes;of stevia and Phytochemicals; its acceptance Medicinal as a significant plant; Steviol;raw material Nutrition; for human Disorders diet. -

In Chemistry, Glycosides Are Certain Molecules in Which a Sugar Part Is
GLYCOSIDES Glycosides may be defined as the organic compounds from plants or animal sources, which on enzymatic or acid hydrolysis give one or more sugar moieties along with non- sugar moiety. Glycosides play numerous important roles in living organisms. Many plants store important chemicals in the form of inactive glycosides; if these chemicals are needed, the glycosides are brought in contact with water and an enzyme, and the sugar part is broken off, making the chemical available for use. Many such plant glycosides are used as medications. In animals (including humans), poisons are often bound to sugar molecules in order to remove them from the body. Formally, a glycoside is any molecule in which a sugar group is bonded through its carbon atom to another group via an O-glycosidic bond or an S-glycosidic bond; glycosides involving the latter are also called thioglycosides. The sugar group is then known as the glycone and the non-sugar group as the aglycone or genin part of the glycoside. The glycone can consist of a single sugar group (monosaccharide) or several sugar groups (oligosaccharide). Classification Classification based on linkages Based on the linkage of sugar moiety to aglycone part 1. O-Glycoside:-Here the sugar is combined with alcoholic or phenolic hydroxyl function of aglycone.eg:-digitalis. 2. N-glycosides:-Here nitrogen of amino group is condensed with a sugar ,eg- Nucleoside 3. S-glycoside:-Here sugar is combined with sulphur of aglycone,eg- isothiocyanate glycosides. 4. C-glycosides:-By condensation of a sugar with a cabon atom, eg-Cascaroside, aloin. Glycosides can be classified by the glycone, by the type of glycosidic bond, and by the aglycone. -

Stevioside from Stevia Rebaudiana Bertoni Increases Insulin Sensitivity in 3T3-L1 Adipocytes
Hindawi Publishing Corporation Evidence-Based Complementary and Alternative Medicine Volume 2013, Article ID 938081, 8 pages http://dx.doi.org/10.1155/2013/938081 Research Article Stevioside from Stevia rebaudiana Bertoni Increases Insulin Sensitivity in 3T3-L1 Adipocytes Nabilatul Hani Mohd-Radzman,1 Wan Iryani Wan Ismail,1 Siti Safura Jaapar,1 Zainah Adam,2 and Aishah Adam1 1 Faculty of Pharmacy, Universiti Teknologi MARA, Puncak Alam Campus, 42300 Bandar Puncak Alam, Selangor, Malaysia 2 Medical Technology Division, Malaysian Nuclear Agency, Bangi, 43000 Kajang, Selangor, Malaysia Correspondence should be addressed to Wan Iryani Wan Ismail; [email protected] Received 19 March 2013; Revised 13 October 2013; Accepted 24 October 2013 Academic Editor: Bechan Sharma Copyright © 2013 Nabilatul Hani Mohd-Radzman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Stevioside from Stevia rebaudiana has been reported to exert antihyperglycemic effects in both rat and human subjects. There have been few studies on these effects in vitro. In this paper, radioactive glucose uptake assay was implemented in order to assess improvements in insulin sensitivity in 3T3-L1 cells by elevation of glucose uptake following treatment with stevioside. Oil Red- O staining and MTT assay were utilized to confirm adipocyte differentiation and cell viability, respectively. Findings from this research showed a significant increase in absorbance values in mature adipocytes following Oil Red-O staining, confirming the differentiation process. Stevioside was noncytotoxic to 3T3-L1 cells as cell viability was reduced by a maximum of 17%, making it impossible to determine its IC50. -

Biorational Preservation of Rose (Rosa Hybrida L.) Cut
Journal of Horticulture and Plant Research Submitted: 2018-05-10 ISSN: 2624-814X, Vol. 4, pp 1-10 Revised: 2018-07-20 doi:10.18052/www.scipress.com/JHPR.4.1 Accepted: 2018-08-08 CC BY 4.0. Published by SciPress Ltd, Switzerland, 2018 Online: 2018-11-15 Biorational Preservation of Rose (Rosa hybrida L.) Cut-Flower Using Stevia (Stevia rebaudiana B.) and Thyme (Thymus vulgaris L.) Extracts John Kamanthi Kiige1,a, Patrick Wachira Mathenge1,b, Agnes Mumo Kavoo2,c* 1Department of Crop Science, Karatina University, Karatina, Kenya 2Department of Horticulture, Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya [email protected], [email protected], *[email protected] Keywords: Floral quality, rose cut flower, stevia extracts, thyme extracts, vase life, Abstract. Rose cut flower is one of the widely grown cut flowers in Kenya. However, most roses have a challenge of short vase life. This study aimed at determining the efficacy of plant extracts from thyme and stevia in preservation of rose cut-flowers. Two rose cut-flower cultivars; ‘radiance and ‘high & sparkling’ were subjected to stevia and thyme extracts each at three levels (0.2, 0.4, and 0.6gL-1). Thyme extracts at a concentration of 0.2 gL-1 significantly (p≤001) extended the vase life of rose cut flower by 3.5 days and floral absorption rates by 10.4% compared to the commercial preservative (chrysal) at the same concentration rates. Application of higher doses (0.4gL-1 and 0.6gL-1) of plant extracts led to shorter vase life (6 days) of rose cut flower and maximum bent neck records at day 8. -
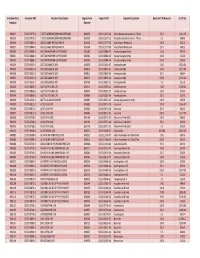
Fagron Item Fagron NDC2 Fagron Description Base Unit of Measure List Price Number Number
Freedom Item Freedom NDC Freedom Description Fagron Item Fagron NDC2 Fagron Description Base Unit of Measure List Price Number Number 900017 52372-7979-02 7-KETO DEHYDROEPIANDROSTERONE 802079 51552-1017-04 Dehydroepiandrosterone, 7-Keto 25 G $163.00 900019 52372-7979-05 7-KETO DEHYDROEPIANDROSTERONE 801939 51552-1017-02 Dehydroepiandrosterone, 7-Keto 5 G $48.00 900026 52372-0846-01 ACESULFAME POTASSIUM NF 802231 51552-1277-05 Acesulfame Potassium 100 G $92.30 900027 52372-0846-03 ACESULFAME POTASSIUM NF 801994 51552-1277-04 Acesulfame Potassium 25 G $38.33 900030 52372-0888-04 ACETAMINOPHEN USP POWDER 801583 51552-0086-07 Acetaminophen Pwd 1 KG $47.00 900031 52372-0888-01 ACETAMINOPHEN USP POWDER 802380 51552-0086-04 Acetaminophen Pwd 100 G $10.00 900032 52372-0888-02 ACETAMINOPHEN USP POWDER 802401 51552-0086-06 Acetaminophen Pwd 500 G $29.00 900034 52372-0651-04 ACETAZOLAMIDE USP 806226 51552-0855-07 Acetazolamide 1 KG $610.00 900035 52372-0651-03 ACETAZOLAMIDE USP 803816 51552-0855-05 Acetazolamide 100 G $225.00 900036 52372-0651-02 ACETAZOLAMIDE USP 803815 51552-0855-04 Acetazolamide 25 G $66.84 900037 52372-0651-05 ACETAZOLAMIDE USP 806227 51552-0855-06 Acetazolamide 500 G $295.00 900038 52372-0651-01 ACETAZOLAMIDE USP 803769 51552-0855-02 Acetazolamide 5 G $22.28 900040 52372-0866-04 ACETYLCYSTEINE USP 801215 51552-0201-07 Acetylcysteine 1 KG $144.00 900041 52372-0866-02 ACETYLCYSTEINE USP 800397 51552-0201-05 Acetylcysteine 100 G $30.00 900043 52372-0866-01 ACETYLCYSTEINE USP 800396 51552-0201-04 Acetylcysteine 25 G $16.00 900046 -

Insight Into the Influence of Grinding on the Extraction Efficiency
applied sciences Article Insight into the Influence of Grinding on the Extraction Efficiency of Selected Bioactive Compounds from Various Plant Leaves Noor Alsaud and Mohammed Farid * Department of Chemical and Materials Engineering, University of Auckland, Private Bag 92019, Auckland 1142, New Zealand; [email protected] * Correspondence: [email protected]; Tel.: +64-9-9234807 Received: 17 August 2020; Accepted: 9 September 2020; Published: 12 September 2020 Abstract: The impact of particle size on bioactive compounds recovery was investigated for two valuable plant matters. This project was to assess the effect of grinding on bioactives extraction of New Zealand Manuka leaves of the Leptospermum scoparium and Stevia rebaudiana plants. Non-grounded and grounded Manuka with particle sizes ranging from 68 µm to 1400 µm were processed using n-hexane extraction for 0–60 min. Moreover, the extraction of two sweeteners from Stevia powder (d 200 µm) was carried out using hot water extraction and Ultrasound as a non-conventional ≤ method. As particle size was reduced, the extraction of bioactives increased, which is expected. However, it was observed that for the small particle size, which was obtained by sever grinding, most of the extraction was achieved as soon as the powder was exposed to the solvent (at zero time) with no further extraction at an extended period. This indicates that short-time exposure appears to be sufficient to recover most of the bioactive compounds, since most of the release of these compounds happened during grinding to fine powder, an issue that has not been addressed in the literature. Keywords: Plant leaves; Particle size; bioactive compounds; Non-conventional extraction; Zero time extraction 1. -

High-Purity Rebaudioside D and Applications Hochreines Rebaudiosid-D Und Anwendungen Rébaudioside D De Grande Pureté Et Applications
(19) TZZ Z_T (11) EP 2 708 548 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07H 1/08 (2006.01) A21D 2/36 (2006.01) 06.12.2017 Bulletin 2017/49 A23G 1/42 (2006.01) (21) Application number: 13196410.8 (22) Date of filing: 13.10.2010 (54) High-Purity Rebaudioside D and Applications Hochreines Rebaudiosid-D und Anwendungen Rébaudioside D de grande pureté et applications (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • Abelyan, Varuzhan GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO 50480 Kuala Lumpur (MY) PL PT RO RS SE SI SK SM TR • Markosyan, Avetik 59200 Kuala Lumpur (MY) (30) Priority: 15.10.2009 US 580233 • Abelyan, Lidia 24.05.2010 US 785501 50480 Kuala Lumpur (MY) 24.05.2010 US 785504 24.05.2010 US 785506 (74) Representative: Hocking, Adrian Niall et al 24.05.2010 US 785507 Albright IP Limited 24.05.2010 US 785508 County House 24.05.2010 US 786392 Bayshill Road 24.05.2010 US 786402 Cheltenham, Glos. GL50 3BA (GB) 24.05.2010 US 786413 24.05.2010 US 786416 (56) References cited: 24.05.2010 US 786427 WO-A1-2009/071277 24.05.2010 US 786430 24.05.2010 US 786419 • I. SAKAMOTO ET AL: "Application of 13C NMR spectroscopy to chemistry of natural glycosices: (43) Date of publication of application: rebaudioside-C,a newsweet diterpeneglycoside 19.03.2014 Bulletin 2014/12 of Stevia rebaudiana", CHEM. -
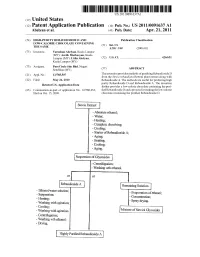
Stevia Extract I
US 20110091637A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0091637 A1 Abelyan et al. (43) Pub. Date: Apr. 21, 2011 (54) HIGH-PURITY REBAUDIOSIDE D AND Publication Classi?cation LOW-CALORIE CHOCOLATE CONTAINING THE SAME (51) Int. Cl. A23G 1/40 (2006.01) (75) Inventors: Varuzhan Abelyan, Kuala Lumpur (MY); Avetik Markosyan, Kuala Lumpur (MY); Lidia Abelyan, (52) US. Cl. ...................................................... .. 426/631 Kuala Lumpur (MY) (73) Assignee: PureCircle Sdn Bhd, Negeri Semb?an (MY) (57) ABSTRACT 21 A 1. N .._ 12/785 507 The invention P rovides methods of P urifyin g RebaudiosideD ( ) pp 0 ’ from the Slevia rebaudiana Bertoni plant extract along With (22) Filed: May 24, 2010 Rebaudioside A. The methods are useful for producing high purity Rebaudioside D and Rebaudioside A. The invention Related US. Application Data further provides a loW-calorie chocolate containing the puri (63) Continuation-in-part of application No, 12/580,233, ?ed Rebaudioside D and a process for making the low-calorie ?led on Oct 15, 2009 chocolate containing the puri?ed Rebaudioside D. Stevia Extract I - Absolute ethanol; - Water; ~ Heating; - Complete dissolving; - Cooling; - Starter of Rebaudioside A; - Aging; - Heating; - Cooling; - Aging. Suspension of Glycosides - centri?lgation; - Washing wth ethanol. or or R?baudloslde A Remaining Solution - Ethanoi/ t ' ' ' ' _ suspensi‘gz. e1 Soiuilon, - Evaporation of ethanol; “ Heating. , - Concentration; - Washmg- _ a with_ agitation;. I ~ Spray drying. - Cooling; . “ Washing with agitation. Mixture of Stevio] Glycosides » Cennifugation; - Washing wth ethanol; ~ Drying. Highly Puri?ed Rebaudioside A Patent Application Publication Apr. 21, 2011 Sheet 2 0f 11 US 2011/0091637 A1 Stevioside FIGZ Patent Application Publication Apr. -

Highly Sweet Compounds of Plant Origin T
glrthi~eS of ~armata[ ge~earth Arch Pharm Res Vol 25, No 6, 725-746, 2002 http://apr.psk.or.kr Highly Sweet Compounds of Plant Origin t Nam-Cheol Kim I and A. Douglas Kinghorn 2 1Chemistry and Life Sciences, Research Triangle Institute, Research Triangle Park, North Carolina, U.S.A. and 2Program for Collaborative Research in the Pharmaceutical Sciences and Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, University of Illinois at Chicago, Chicago, Illinois, U.S.A. (Received October 18, 2002) The demand for new alternative "low calorie" sweeteners for dietetic and diabetic purposes has increased worldwide. Although the currently developed and commercially used highly sweet sucrose substitutes are mostly synthetic compounds, the search for such compounds from natural sources is continuing. As of mid-2002, over 100 plant-derived sweet compounds of 20 major structural types had been reported, and were isolated from more than 25 different families of green plants. Several of these highly sweet natural products are marketed as sweeteners or flavoring agents in some countries as pure compounds, compound mixtures, or refined extracts. These highly sweet natural substances are reviewed herein. Key words: Low-Calorie Natural Sweeteners, Plants, Glycyrrhizin, Mogroside V, Rebaudio- side A, Stevioside, Thaumatin, Terpenoids, Steroids, Flavonoids, Proteins INTRODUCTION Most of the currently available potently sweet, low calorie sucrose substitutes in the world market are synthetic com- The consumption of sucrose as a sweetener has been pounds, inclusive of acesulfame-K, alitame, aspartame, associated with several nutritional and medical problems, cyclamate, saccharin, and sucralose (Duffy and Anderson, with dental caries being the most widely described (Grenby, 1998).