Salt Stress Mitigation Via the Foliar Application of Chitosan-Functionalized Selenium and Anatase Titanium Dioxide Nanoparticles in Stevia (Stevia Rebaudiana Bertoni)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FEMA GRAS 29 December 2019 SUPPLEMENTARY INFORMATION 1
SUPPLEMENTARY INFORMATION 1: Identity for Natural Flavor Complexes as Evaluated by the Expert Panel The Identification Description as Reviewed by the FEMA FEMA No.1 FEMA Primary Name Expert Panel Rebaudioside M ≥80%; Rebaudioside D 5-20%; Total 4895 Rebaudioside M steviol glycosides ≥95%. Glutamic acid 35-40%; Other amino acids 1-2%; Total Corynebacterium glutamicum corn nitrogen 6-7%; Aliphatic primary alcohols, aldehydes, 4907 syrup fermentation product carboxylic acids, acetals and esters containing additional oxygenated functional groups 1-2%; Minerals 9-11% Inosine 5´-monophosphate 20-25%; Amino acids 7-8%; Corynebacterium stationis corn 4908 Minerals 23-25%; water 28-37%; Other nucleotides 1-2%; syrup fermentation product Total nitrogen 5-8% Supraglucosylated steviol glycosides 70-80%; Rebaudioside Glucosylated steviol glycosides, 4909 A 14-20%; Steviol glycosides not further glucosylated, each 70-80% individually, not to exceed 3%; Maltodextrin 3-10% Supraglucosylated steviol glycosides 30-40%; Rebaudioside Glucosylated steviol glycosides, A 5-8%; Not more than 4% stevioside; All other individual 4910 40% steviol glycosides not further glucosylated <3%; Maltodextrin 45-60% Stevioside 70-80%; Rebaudioside A 13-18%; Steviobioside 1- 3%; Rebaudioside C 2-3%; Total glycosides (including 4911 Stevia extract stevioside, 70% Rebaudioside D, Rebaudioside B, Rebaudioside F, Dulcoside A, and Rubusoside) <3% Derived from hibiscus blossom calyces (Hibiscus sabdariffa L.) , Hibiscus blossom extract is measured as water 30-60%; 4912 Hibiscus -

Beneficial Effects of Stevioside on Ages, Blood Glucose, Lipid Profile and Renal Status in Streptozotocin-Induced Diabetic Rats
J Appl Biomed journal homepage: http://jab.zsf.jcu.cz DOI: 10.32725/jab.2019.013 Journal of Applied Biomedicine Original research article Beneficial effects of Stevioside on AGEs, blood glucose, lipid profile and renal status in streptozotocin-induced diabetic rats Urmila Aswar 1 *, Vinayak Gogawale 2, Pankaj Miniyar 2, Yugendra Patil 3 1 Bharati Vidyapeeth (Deemed to be University), Poona College of Pharmacy, Erandwane, Pune, Maharashtra, India 2 STES’s Sinhgad Institute of Pharmacy, Savitribai Phule Pune University, Narhe, Pune, Maharashtra, India 3 National Chemical Laboratory, Pune, Maharashtra, India Abstract The advanced glycated end products (AGEs) are formed in the diabetic patients; it is a major cause of macrovascular and microvascular complications in diabetes. Clinically there is no treatment available for the AGEs. Stveoside (Stv), a sweetener has potent anti-diabetic and anti-oxidant activity. Hence, we investigated its use in prevention of AGEs formation using in vitro and in vivo models. Diabetes was induced by streptozotocin (STZ). These rats were kept without treatment till blood HbA1c was markedly increased. They were then divided into 5 groups and treated orally with vehicle or Metformin (MET) or Stv respectively for 28 days. Every 7th day, animals were tested for body weight and blood glucose (BG). On the last day of treatment, all the groups were evaluated for physiological and biochemical parameters, histopathology and AGEs; N-carboxymethyl-lysine (CML) estimation. Stv showed inhibition of AGEs in in vitro as well as in in vivo respectively. Positive effects were seen on the BG, lipid profile and urine parameters as well it showed reduced formation of CML. -

Morpho-Anatomical Study of Stevia Rebaudiana Roots Grown in Vitro and in Vivo
Revista Brasileira de Farmacognosia 27 (2017) 34–39 ww w.elsevier.com/locate/bjp Original Article Morpho-anatomical study of Stevia rebaudiana roots grown in vitro and in vivo a a a b c Rafael V. Reis , Talita P.C. Chierrito , Thaila F.O. Silva , Adriana L.M. Albiero , Luiz A. Souza , d a,b a,b,∗ José E. Gonc¸ alves , Arildo J.B. Oliveira , Regina A.C. Gonc¸ alves a Programa de Pós-graduac¸ ão em Ciências Farmacêuticas, Universidade Estadual de Maringá, Maringá, PR, Brazil b Departamento de Farmácia, Universidade Estadual de Maringá, Campus Universitário, Maringá, PR, Brazil c Departamento de Biologia, Universidade Estadual de Maringá, Campus Universitário, Maringá, PR, Brazil d Programa de Mestrado em Promoc¸ ão da Saúde, Centro Universitário de Maringá, Maringá, PR, Brazil a r a b s t r a c t t i c l e i n f o Article history: Stevia rebaudiana (Bertoni) Bertoni, Asteraceae, is used as a food additive because its leaves are a source Received 16 May 2016 of steviol glycosides. There are examples of tissue culture based on micropropagation and phytochemical Accepted 14 August 2016 production of S. rebaudiana leaves but there are few studies on adventitious root culture of S. rebaudiana. Available online 7 October 2016 More than 90% of the plants used in industry are harvested indiscriminately. In order to overcome this situation, the development of methodologies that employ biotechnology, such as root culture, provides Keywords: suitable alternatives for the sustainable use of plants. The aim of this study was to compare morpho- Stevia rebaudiana anatomical transverse sections of S. -

FEMA GRAS 29 December 2019 SUPPLEMENTARY INFORMATION 1
SUPPLEMENTARY INFORMATION 1: Identity for Natural Flavor Complexes as Evaluated by the Expert Panel The Identification Description as Reviewed by the FEMA FEMA No.1 FEMA Primary Name Expert Panel Rebaudioside M ≥80%; Rebaudioside D 5-20%; Total 4895 Rebaudioside M steviol glycosides ≥95%. Glutamic acid 35-40%; Other amino acids 1-2%; Total Corynebacterium glutamicum corn nitrogen 6-7%; Aliphatic primary alcohols, aldehydes, 4907 syrup fermentation product carboxylic acids, acetals and esters containing additional oxygenated functional groups 1-2%; Minerals 9-11% Inosine 5´-monophosphate 20-25%; Amino acids 7-8%; Corynebacterium stationis corn 4908 Minerals 23-25%; water 28-37%; Other nucleotides 1-2%; syrup fermentation product Total nitrogen 5-8% Supraglucosylated steviol glycosides 70-80%; Rebaudioside Glucosylated steviol glycosides, 4909 A 14-20%; Steviol glycosides not further glucosylated, each 70-80% individually, not to exceed 3%; Maltodextrin 3-10% Supraglucosylated steviol glycosides 30-40%; Rebaudioside Glucosylated steviol glycosides, A 5-8%; Not more than 4% stevioside; All other individual 4910 40% steviol glycosides not further glucosylated <3%; Maltodextrin 45-60% Stevioside 70-80%; Rebaudioside A 13-18%; Steviobioside 1- 3%; Rebaudioside C 2-3%; Total glycosides (including 4911 Stevia extract stevioside, 70% Rebaudioside D, Rebaudioside B, Rebaudioside F, Dulcoside A, and Rubusoside) <3% Derived from hibiscus blossom calyces (Hibiscus sabdariffa L.) , Hibiscus blossom extract is measured as water 30-60%; 4912 Hibiscus -

Nutritional and Medicinal Properties of Stevia Rebaudiana
Review Article Curr Res Diabetes Obes J Volume 13 Issue 4 - July 2020 Copyright © All rights are reserved by Fasiha Ahsan DOI: 10.19080/CRDOJ.2020.13.555867 Nutritional and Medicinal Properties of Stevia Rebaudiana Fasiha Ahsan*, Shahid Bashir and Faiz-ul-Hassan Shah University Institute of Diet and Nutritional Sciences, The University of Lahore, Pakistan Submission: June 25, 2020; Published: July 16, 2020 *Corresponding author: Fasiha Ahsan, PhD Scholar, University Institute of Diet and Nutritional Sciences, The University of Lahore, Pakistan Abstract Researches on new molecules with the least toxic effects and better potency is on its way and more attention is being given upon medicinal plants for forcing away the above problems. Medicinal plants have been recognized as potential drug candidates. Stevia, a natural sweetener with medicinal properties and also having nutritional, therapeutic and industrial importance is being used all over the world. Stevia rebaudiana leaves are usually referred to as candy, sweet and honey leaves. Diterpene glycosides are responsible for its high sweetening potential of leaves. The phytochemical properties of bioactive chemicals present in stevia leaves are involves in maintaining the physiological functions of human body. Paper also highlights the importance of nutritional aspects of dried stevia leaves, metabolism of stevia, effects of it consumption on human health and clinical studies related to stevia ingestion. Various medicinal properties of stevia leaves discussed in paper like anti-hyperglycemia, anti-oxidative, hypotensive, nephro-protective, hepato protective, antibacterial and antifungal. Basic purpose of this review to understand the medicinalKeywords: potential Stevia; Diabetes;of stevia and Phytochemicals; its acceptance Medicinal as a significant plant; Steviol;raw material Nutrition; for human Disorders diet. -

In Chemistry, Glycosides Are Certain Molecules in Which a Sugar Part Is
GLYCOSIDES Glycosides may be defined as the organic compounds from plants or animal sources, which on enzymatic or acid hydrolysis give one or more sugar moieties along with non- sugar moiety. Glycosides play numerous important roles in living organisms. Many plants store important chemicals in the form of inactive glycosides; if these chemicals are needed, the glycosides are brought in contact with water and an enzyme, and the sugar part is broken off, making the chemical available for use. Many such plant glycosides are used as medications. In animals (including humans), poisons are often bound to sugar molecules in order to remove them from the body. Formally, a glycoside is any molecule in which a sugar group is bonded through its carbon atom to another group via an O-glycosidic bond or an S-glycosidic bond; glycosides involving the latter are also called thioglycosides. The sugar group is then known as the glycone and the non-sugar group as the aglycone or genin part of the glycoside. The glycone can consist of a single sugar group (monosaccharide) or several sugar groups (oligosaccharide). Classification Classification based on linkages Based on the linkage of sugar moiety to aglycone part 1. O-Glycoside:-Here the sugar is combined with alcoholic or phenolic hydroxyl function of aglycone.eg:-digitalis. 2. N-glycosides:-Here nitrogen of amino group is condensed with a sugar ,eg- Nucleoside 3. S-glycoside:-Here sugar is combined with sulphur of aglycone,eg- isothiocyanate glycosides. 4. C-glycosides:-By condensation of a sugar with a cabon atom, eg-Cascaroside, aloin. Glycosides can be classified by the glycone, by the type of glycosidic bond, and by the aglycone. -

Steviol Glycosides from Stevia Rebaudiana Bertoni
0 out of 21 Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 84th meeting 2017 Steviol Glycosides from Stevia rebaudiana Bertoni This monograph was also published in: Compendium of Food Additive Specifications. Joint FAO/WHO Expert Committee on Food Additives (JECFA), 84th meeting 2017. FAO JECFA Monographs 20 © FAO/WHO 2017 1 out of 21 STEVIOL GLYCOSIDES FROM STEVIA REBAUDIANA BERTONI Prepared at the 84th JECFA (2017) and published in FAO JECFA Monographs 20 (2017), superseding tentative specifications prepared at the 82nd JECFA (2016) and published in FAO JECFA Monographs 19 (2016). An ADI of 0 - 4 mg/kg bw (expressed as steviol) was established at the 69th JECFA (2008). SYNONYMS INS No. 960 DEFINITION Steviol glycosides consist of a mixture of compounds containing a steviol backbone conjugated to any number or combination of the principal sugar moieties (glucose, rhamnose, xylose, fructose, arabinose, galactose and deoxyglucose) in any of the orientations occurring in the leaves of Stevia rebaudiana Bertoni. The product is obtained from the leaves of Stevia rebaudiana Bertoni. The leaves are extracted with hot water and the aqueous extract is passed through an adsorption resin to trap and concentrate the component steviol glycosides. The resin is washed with a solvent alcohol to release the glycosides and the product is recrystallized from methanol or aqueous ethanol. Ion exchange resins may be used in the purification process. The final product may be spray-dried. Chemical name See Appendix 1 C.A.S. number See Appendix 1 Chemical formula See Appendix 1 Structural formula Steviol (R1 = R2 = H) is the aglycone of the steviol glycosides. -

Rapid and Economic Determination of 13 Steviol Glycosides In
pubs.acs.org/JAFC Article Rapid and Economic Determination of 13 Steviol Glycosides in Market-Available Food, Dietary Supplements, and Ingredients: Single-Laboratory Validation of an HPLC Method Zhiyan Liu, Kangzi Ren, Ye Feng, Tommy Uong, Scott Krepich, and Hong You* Cite This: https://dx.doi.org/10.1021/acs.jafc.0c03453 Read Online ACCESS Metrics & More Article Recommendations *sı Supporting Information ABSTRACT: Steviol glycosides, obtained from leaves of Stevia rebaudiana Bertoni (stevia) or produced via bioconversion and biosynthesis, are diterpenes used by the food/dietary supplement industry as zero-calorie sweeteners derived from natural sources. JECFA 2017 is the most updated international standardized method but it runs for 80 min per sample with suboptimal separations on several critical pairs for its high-performance liquid chromatography-ultraviolet (HPLC-UV) determination. We developed and validated a rapid and economic HPLC-UV method using the superficially porous particle column to determine 13 steviol glycosides (stevioside, dulcoside A, rubusoside, steviobioside, and rebaudioside A−F, I, M, and N). Baseline separation with a minimum resolution of 1.5 for 13 steviol glycosides was achieved within only 14 min of separation time. The hydrocarbon stationary phase with additional steric interactions from the isobutyl side chains on the C18 ligand was shown to be an important contributor to chromatographic selectivity of several critical pairs of steviol glycosides. The method was proven to perform suitably on columns from three different manufacturers and two HPLC instruments. The method was further used to perform a single-lab validation on eight food and supplement products with multiple matrices. The results ranged from 0.05% w/w rebaudioside A for a hard-candy finished product to 100.8% w/w purity for a rebaudioside M raw ingredient. -

Synthesis and Pharmacological Screening of Novel 1,5
Vol 2, Issue 1 , 2014 Review Article STEVIOL GLYCOSIDES AND THEIR USE IN FOOD PROCESSING: A REVIEW ROLLY MEHROTRA*1, DHEER SINGH2, AVINASH TIWARI3 1 PhD Student, Centre for Food Technology, Jiwaji University, Gwalior (MP)., 1 Dean, Institute of Engineering and Technology, Bundelkhand University, Jhansi ³ Reader, department of Botany, Jiwaji University, Gwalior (MP) Email: tiwariavinash2@ gmail.com Received: 29 June 2014, Revised and Accepted: 7 August 2014 ABSTRACT Steviol glycosides are the proteinacious secondary metabolites present in the leaves of Stevia rebaudiana Bertoni. These SG’s are the principal sweetening agents which are 200-300 times more sweeter than sucrose. A number of SG’s are present in the leaves of Stevia namely, stevioside, dulcoside A, rebaudioside A, rebaudioside B, rebaudioside C (previously known as dulcoside B), rebaudioside D, rebaudioside E and steviolbioside, 100–125. At the 63rd meeting a temporary ADI of 0-2 mg/kg bodyweight and day, expressed as steviol, was established based on a No-Observed- Effect-Level (NOEL). Steviol glycoside extracts have broad applications as sweetener in the manufacture of fruit and milk drinks, desserts, yoghurt, delicacies, confectioneries, fruit products, processed seafood products, pickles, table-top sweeteners and dietary supplements. Other aspects considered are physicochemical and biologic properties for food processing, stability and therapeutic value of SG’s. nutritional information, Stevia market and household uses. Keywords: Stevia rebaudiana, Steviol glycosides, stevioside and rebaudioside. INTRODUCTION Stevia rebaudiana, a perennial herb from the Asteraceae family, is dulcoside. The two primary compounds, stevioside and rebaudioside known to the scientific world for its sweetness and steviol glycosides A, use only glucose: stevioside has two linked glucose molecules at (SGs). -

Stevioside from Stevia Rebaudiana Bertoni Increases Insulin Sensitivity in 3T3-L1 Adipocytes
Hindawi Publishing Corporation Evidence-Based Complementary and Alternative Medicine Volume 2013, Article ID 938081, 8 pages http://dx.doi.org/10.1155/2013/938081 Research Article Stevioside from Stevia rebaudiana Bertoni Increases Insulin Sensitivity in 3T3-L1 Adipocytes Nabilatul Hani Mohd-Radzman,1 Wan Iryani Wan Ismail,1 Siti Safura Jaapar,1 Zainah Adam,2 and Aishah Adam1 1 Faculty of Pharmacy, Universiti Teknologi MARA, Puncak Alam Campus, 42300 Bandar Puncak Alam, Selangor, Malaysia 2 Medical Technology Division, Malaysian Nuclear Agency, Bangi, 43000 Kajang, Selangor, Malaysia Correspondence should be addressed to Wan Iryani Wan Ismail; [email protected] Received 19 March 2013; Revised 13 October 2013; Accepted 24 October 2013 Academic Editor: Bechan Sharma Copyright © 2013 Nabilatul Hani Mohd-Radzman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Stevioside from Stevia rebaudiana has been reported to exert antihyperglycemic effects in both rat and human subjects. There have been few studies on these effects in vitro. In this paper, radioactive glucose uptake assay was implemented in order to assess improvements in insulin sensitivity in 3T3-L1 cells by elevation of glucose uptake following treatment with stevioside. Oil Red- O staining and MTT assay were utilized to confirm adipocyte differentiation and cell viability, respectively. Findings from this research showed a significant increase in absorbance values in mature adipocytes following Oil Red-O staining, confirming the differentiation process. Stevioside was noncytotoxic to 3T3-L1 cells as cell viability was reduced by a maximum of 17%, making it impossible to determine its IC50. -

Biorational Preservation of Rose (Rosa Hybrida L.) Cut
Journal of Horticulture and Plant Research Submitted: 2018-05-10 ISSN: 2624-814X, Vol. 4, pp 1-10 Revised: 2018-07-20 doi:10.18052/www.scipress.com/JHPR.4.1 Accepted: 2018-08-08 CC BY 4.0. Published by SciPress Ltd, Switzerland, 2018 Online: 2018-11-15 Biorational Preservation of Rose (Rosa hybrida L.) Cut-Flower Using Stevia (Stevia rebaudiana B.) and Thyme (Thymus vulgaris L.) Extracts John Kamanthi Kiige1,a, Patrick Wachira Mathenge1,b, Agnes Mumo Kavoo2,c* 1Department of Crop Science, Karatina University, Karatina, Kenya 2Department of Horticulture, Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya [email protected], [email protected], *[email protected] Keywords: Floral quality, rose cut flower, stevia extracts, thyme extracts, vase life, Abstract. Rose cut flower is one of the widely grown cut flowers in Kenya. However, most roses have a challenge of short vase life. This study aimed at determining the efficacy of plant extracts from thyme and stevia in preservation of rose cut-flowers. Two rose cut-flower cultivars; ‘radiance and ‘high & sparkling’ were subjected to stevia and thyme extracts each at three levels (0.2, 0.4, and 0.6gL-1). Thyme extracts at a concentration of 0.2 gL-1 significantly (p≤001) extended the vase life of rose cut flower by 3.5 days and floral absorption rates by 10.4% compared to the commercial preservative (chrysal) at the same concentration rates. Application of higher doses (0.4gL-1 and 0.6gL-1) of plant extracts led to shorter vase life (6 days) of rose cut flower and maximum bent neck records at day 8. -
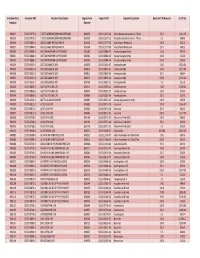
Fagron Item Fagron NDC2 Fagron Description Base Unit of Measure List Price Number Number
Freedom Item Freedom NDC Freedom Description Fagron Item Fagron NDC2 Fagron Description Base Unit of Measure List Price Number Number 900017 52372-7979-02 7-KETO DEHYDROEPIANDROSTERONE 802079 51552-1017-04 Dehydroepiandrosterone, 7-Keto 25 G $163.00 900019 52372-7979-05 7-KETO DEHYDROEPIANDROSTERONE 801939 51552-1017-02 Dehydroepiandrosterone, 7-Keto 5 G $48.00 900026 52372-0846-01 ACESULFAME POTASSIUM NF 802231 51552-1277-05 Acesulfame Potassium 100 G $92.30 900027 52372-0846-03 ACESULFAME POTASSIUM NF 801994 51552-1277-04 Acesulfame Potassium 25 G $38.33 900030 52372-0888-04 ACETAMINOPHEN USP POWDER 801583 51552-0086-07 Acetaminophen Pwd 1 KG $47.00 900031 52372-0888-01 ACETAMINOPHEN USP POWDER 802380 51552-0086-04 Acetaminophen Pwd 100 G $10.00 900032 52372-0888-02 ACETAMINOPHEN USP POWDER 802401 51552-0086-06 Acetaminophen Pwd 500 G $29.00 900034 52372-0651-04 ACETAZOLAMIDE USP 806226 51552-0855-07 Acetazolamide 1 KG $610.00 900035 52372-0651-03 ACETAZOLAMIDE USP 803816 51552-0855-05 Acetazolamide 100 G $225.00 900036 52372-0651-02 ACETAZOLAMIDE USP 803815 51552-0855-04 Acetazolamide 25 G $66.84 900037 52372-0651-05 ACETAZOLAMIDE USP 806227 51552-0855-06 Acetazolamide 500 G $295.00 900038 52372-0651-01 ACETAZOLAMIDE USP 803769 51552-0855-02 Acetazolamide 5 G $22.28 900040 52372-0866-04 ACETYLCYSTEINE USP 801215 51552-0201-07 Acetylcysteine 1 KG $144.00 900041 52372-0866-02 ACETYLCYSTEINE USP 800397 51552-0201-05 Acetylcysteine 100 G $30.00 900043 52372-0866-01 ACETYLCYSTEINE USP 800396 51552-0201-04 Acetylcysteine 25 G $16.00 900046