Biochemical Studies on the Production of Active Constituents in Stevia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ruta Graveolens L. Essential Oil Composition Under Different Nutritional Treatments
American-Eurasian J. Agric. & Environ. Sci., 13 (10): 1390-1395, 2013 ISSN 1818-6769 © IDOSI Publications, 2013 DOI: 10.5829/idosi.aejaes.2013.13.10.11248 Ruta graveolens L. Essential Oil Composition under Different Nutritional Treatments 12Afaq Ahmad Malik, Showkat R. Mir and 1Javed Ahmad 1Department of Botany, Jamia Hamdard, New Delhi 110062, India 2Department of Pharmacognosy and Phytochemistry, Jamia Hamdard, New Delhi 110 062, India Abstract: The use of un-exploited organic industrial by-products and municipal wastes as soil organic amendment has an economic value and environmental interest. However, little is known about their effectiveness on medicinal plants cultivation. An experiment was conducted in this regard to assess the impact of farmyard manure (FYM), composted sugarcane pressmud (CPM) and sewage sludge biosolid (SSB) on volatile oil composition of Ruta graveolens L., an important aromatic medicinal herb used frequently in Unani system of medicine in India. Volatile oil in the aerial parts of the plant was isolated by hydro-distillation and analyzed by GC-MS. Hydro-distillation of untreated (control) plants yielded 0.32% essential oil on fresh weight basis. The predominant components in the essential oil were n-Hex-4-en-3-one (55.06%), n-Pent-3-one (28.17%), n-Hex-3-en-2-one (14.07%) and n-Hex-5-en-3-one (0.67%). Essential oil obtained from plants treated with FYM amounted to 0.36% of fresh weight and consisted mainly of n-Hex-4-en-3-one (53.64%), n-Pent-3-one (37.82%) and n-Hex-3-en-2-one (7.22%). -

FEMA GRAS 29 December 2019 SUPPLEMENTARY INFORMATION 1
SUPPLEMENTARY INFORMATION 1: Identity for Natural Flavor Complexes as Evaluated by the Expert Panel The Identification Description as Reviewed by the FEMA FEMA No.1 FEMA Primary Name Expert Panel Rebaudioside M ≥80%; Rebaudioside D 5-20%; Total 4895 Rebaudioside M steviol glycosides ≥95%. Glutamic acid 35-40%; Other amino acids 1-2%; Total Corynebacterium glutamicum corn nitrogen 6-7%; Aliphatic primary alcohols, aldehydes, 4907 syrup fermentation product carboxylic acids, acetals and esters containing additional oxygenated functional groups 1-2%; Minerals 9-11% Inosine 5´-monophosphate 20-25%; Amino acids 7-8%; Corynebacterium stationis corn 4908 Minerals 23-25%; water 28-37%; Other nucleotides 1-2%; syrup fermentation product Total nitrogen 5-8% Supraglucosylated steviol glycosides 70-80%; Rebaudioside Glucosylated steviol glycosides, 4909 A 14-20%; Steviol glycosides not further glucosylated, each 70-80% individually, not to exceed 3%; Maltodextrin 3-10% Supraglucosylated steviol glycosides 30-40%; Rebaudioside Glucosylated steviol glycosides, A 5-8%; Not more than 4% stevioside; All other individual 4910 40% steviol glycosides not further glucosylated <3%; Maltodextrin 45-60% Stevioside 70-80%; Rebaudioside A 13-18%; Steviobioside 1- 3%; Rebaudioside C 2-3%; Total glycosides (including 4911 Stevia extract stevioside, 70% Rebaudioside D, Rebaudioside B, Rebaudioside F, Dulcoside A, and Rubusoside) <3% Derived from hibiscus blossom calyces (Hibiscus sabdariffa L.) , Hibiscus blossom extract is measured as water 30-60%; 4912 Hibiscus -

Physiological Responses of Ocimum Basilicum, Salvia Officinalis, And
plants Article Physiological Responses of Ocimum basilicum, Salvia officinalis, and Mentha piperita to Leaf Wounding Konstantinos Vrakas, Efterpi Florou, Athanasios Koulopoulos and George Zervoudakis * Department of Agriculture, University of Patras, Terma Theodoropoulou, 27200 Amaliada, Greece; [email protected] (K.V.); evtefl[email protected] (E.F.); [email protected] (A.K.) * Correspondence: [email protected] Abstract: The investigation about the leaf wounding effect on plant physiological procedures and on leaf pigments content will contribute to the understanding of the plants’ responses against this abiotic stress. During the experiment, some physiological parameters such as photosynthesis, transpiration and stomatal conductance as well as the chlorophyll and anthocyanin leaf contents of Ocimum basilicum, Salvia officinalis, and Mentha piperita plants were measured for about 20–40 days. All the measurements were conducted on control and wounded plants while in the latter, they were conducted on both wounded and intact leaves. A wide range of responses was observed in the wounded leaves, that is: (a) immediate decrease of the gas exchange parameters and long-term decrease of almost all the measured variables from O. basilicum, (b) immediate but only short-term decrease of the gas exchange parameters and no effect on pigments from M. piperita, and (c) no effect on the gas exchange parameters and decrease of the pigments content from S. officinalis. Regarding the intact leaves, in general, they exhibited a similar profile with the control ones for all plants. These results imply that the plant response to wounding is a complex phenomenon depending on plant species and the severity of the injury. Citation: Vrakas, K.; Florou, E.; Koulopoulos, A.; Zervoudakis, G. -

Beneficial Effects of Stevioside on Ages, Blood Glucose, Lipid Profile and Renal Status in Streptozotocin-Induced Diabetic Rats
J Appl Biomed journal homepage: http://jab.zsf.jcu.cz DOI: 10.32725/jab.2019.013 Journal of Applied Biomedicine Original research article Beneficial effects of Stevioside on AGEs, blood glucose, lipid profile and renal status in streptozotocin-induced diabetic rats Urmila Aswar 1 *, Vinayak Gogawale 2, Pankaj Miniyar 2, Yugendra Patil 3 1 Bharati Vidyapeeth (Deemed to be University), Poona College of Pharmacy, Erandwane, Pune, Maharashtra, India 2 STES’s Sinhgad Institute of Pharmacy, Savitribai Phule Pune University, Narhe, Pune, Maharashtra, India 3 National Chemical Laboratory, Pune, Maharashtra, India Abstract The advanced glycated end products (AGEs) are formed in the diabetic patients; it is a major cause of macrovascular and microvascular complications in diabetes. Clinically there is no treatment available for the AGEs. Stveoside (Stv), a sweetener has potent anti-diabetic and anti-oxidant activity. Hence, we investigated its use in prevention of AGEs formation using in vitro and in vivo models. Diabetes was induced by streptozotocin (STZ). These rats were kept without treatment till blood HbA1c was markedly increased. They were then divided into 5 groups and treated orally with vehicle or Metformin (MET) or Stv respectively for 28 days. Every 7th day, animals were tested for body weight and blood glucose (BG). On the last day of treatment, all the groups were evaluated for physiological and biochemical parameters, histopathology and AGEs; N-carboxymethyl-lysine (CML) estimation. Stv showed inhibition of AGEs in in vitro as well as in in vivo respectively. Positive effects were seen on the BG, lipid profile and urine parameters as well it showed reduced formation of CML. -

Companion Plants for Better Yields
Companion Plants for Better Yields PLANT COMPATIBLE INCOMPATIBLE Angelica Dill Anise Coriander Carrot Black Walnut Tree, Apple Hawthorn Basil, Carrot, Parsley, Asparagus Tomato Azalea Black Walnut Tree Barberry Rye Barley Lettuce Beans, Broccoli, Brussels Sprouts, Cabbage, Basil Cauliflower, Collard, Kale, Rue Marigold, Pepper, Tomato Borage, Broccoli, Cabbage, Carrot, Celery, Chinese Cabbage, Corn, Collard, Cucumber, Eggplant, Irish Potato, Beet, Chive, Garlic, Onion, Beans, Bush Larkspur, Lettuce, Pepper Marigold, Mint, Pea, Radish, Rosemary, Savory, Strawberry, Sunflower, Tansy Basil, Borage, Broccoli, Carrot, Chinese Cabbage, Corn, Collard, Cucumber, Eggplant, Beet, Garlic, Onion, Beans, Pole Lettuce, Marigold, Mint, Kohlrabi Pea, Radish, Rosemary, Savory, Strawberry, Sunflower, Tansy Bush Beans, Cabbage, Beets Delphinium, Onion, Pole Beans Larkspur, Lettuce, Sage PLANT COMPATIBLE INCOMPATIBLE Beans, Squash, Borage Strawberry, Tomato Blackberry Tansy Basil, Beans, Cucumber, Dill, Garlic, Hyssop, Lettuce, Marigold, Mint, Broccoli Nasturtium, Onion, Grapes, Lettuce, Rue Potato, Radish, Rosemary, Sage, Thyme, Tomato Basil, Beans, Dill, Garlic, Hyssop, Lettuce, Mint, Brussels Sprouts Grapes, Rue Onion, Rosemary, Sage, Thyme Basil, Beets, Bush Beans, Chamomile, Celery, Chard, Dill, Garlic, Grapes, Hyssop, Larkspur, Lettuce, Cabbage Grapes, Rue Marigold, Mint, Nasturtium, Onion, Rosemary, Rue, Sage, Southernwood, Spinach, Thyme, Tomato Plant throughout garden Caraway Carrot, Dill to loosen soil Beans, Chive, Delphinium, Pea, Larkspur, Lettuce, -

Morpho-Anatomical Study of Stevia Rebaudiana Roots Grown in Vitro and in Vivo
Revista Brasileira de Farmacognosia 27 (2017) 34–39 ww w.elsevier.com/locate/bjp Original Article Morpho-anatomical study of Stevia rebaudiana roots grown in vitro and in vivo a a a b c Rafael V. Reis , Talita P.C. Chierrito , Thaila F.O. Silva , Adriana L.M. Albiero , Luiz A. Souza , d a,b a,b,∗ José E. Gonc¸ alves , Arildo J.B. Oliveira , Regina A.C. Gonc¸ alves a Programa de Pós-graduac¸ ão em Ciências Farmacêuticas, Universidade Estadual de Maringá, Maringá, PR, Brazil b Departamento de Farmácia, Universidade Estadual de Maringá, Campus Universitário, Maringá, PR, Brazil c Departamento de Biologia, Universidade Estadual de Maringá, Campus Universitário, Maringá, PR, Brazil d Programa de Mestrado em Promoc¸ ão da Saúde, Centro Universitário de Maringá, Maringá, PR, Brazil a r a b s t r a c t t i c l e i n f o Article history: Stevia rebaudiana (Bertoni) Bertoni, Asteraceae, is used as a food additive because its leaves are a source Received 16 May 2016 of steviol glycosides. There are examples of tissue culture based on micropropagation and phytochemical Accepted 14 August 2016 production of S. rebaudiana leaves but there are few studies on adventitious root culture of S. rebaudiana. Available online 7 October 2016 More than 90% of the plants used in industry are harvested indiscriminately. In order to overcome this situation, the development of methodologies that employ biotechnology, such as root culture, provides Keywords: suitable alternatives for the sustainable use of plants. The aim of this study was to compare morpho- Stevia rebaudiana anatomical transverse sections of S. -

FEMA GRAS 29 December 2019 SUPPLEMENTARY INFORMATION 1
SUPPLEMENTARY INFORMATION 1: Identity for Natural Flavor Complexes as Evaluated by the Expert Panel The Identification Description as Reviewed by the FEMA FEMA No.1 FEMA Primary Name Expert Panel Rebaudioside M ≥80%; Rebaudioside D 5-20%; Total 4895 Rebaudioside M steviol glycosides ≥95%. Glutamic acid 35-40%; Other amino acids 1-2%; Total Corynebacterium glutamicum corn nitrogen 6-7%; Aliphatic primary alcohols, aldehydes, 4907 syrup fermentation product carboxylic acids, acetals and esters containing additional oxygenated functional groups 1-2%; Minerals 9-11% Inosine 5´-monophosphate 20-25%; Amino acids 7-8%; Corynebacterium stationis corn 4908 Minerals 23-25%; water 28-37%; Other nucleotides 1-2%; syrup fermentation product Total nitrogen 5-8% Supraglucosylated steviol glycosides 70-80%; Rebaudioside Glucosylated steviol glycosides, 4909 A 14-20%; Steviol glycosides not further glucosylated, each 70-80% individually, not to exceed 3%; Maltodextrin 3-10% Supraglucosylated steviol glycosides 30-40%; Rebaudioside Glucosylated steviol glycosides, A 5-8%; Not more than 4% stevioside; All other individual 4910 40% steviol glycosides not further glucosylated <3%; Maltodextrin 45-60% Stevioside 70-80%; Rebaudioside A 13-18%; Steviobioside 1- 3%; Rebaudioside C 2-3%; Total glycosides (including 4911 Stevia extract stevioside, 70% Rebaudioside D, Rebaudioside B, Rebaudioside F, Dulcoside A, and Rubusoside) <3% Derived from hibiscus blossom calyces (Hibiscus sabdariffa L.) , Hibiscus blossom extract is measured as water 30-60%; 4912 Hibiscus -

Nutritional and Medicinal Properties of Stevia Rebaudiana
Review Article Curr Res Diabetes Obes J Volume 13 Issue 4 - July 2020 Copyright © All rights are reserved by Fasiha Ahsan DOI: 10.19080/CRDOJ.2020.13.555867 Nutritional and Medicinal Properties of Stevia Rebaudiana Fasiha Ahsan*, Shahid Bashir and Faiz-ul-Hassan Shah University Institute of Diet and Nutritional Sciences, The University of Lahore, Pakistan Submission: June 25, 2020; Published: July 16, 2020 *Corresponding author: Fasiha Ahsan, PhD Scholar, University Institute of Diet and Nutritional Sciences, The University of Lahore, Pakistan Abstract Researches on new molecules with the least toxic effects and better potency is on its way and more attention is being given upon medicinal plants for forcing away the above problems. Medicinal plants have been recognized as potential drug candidates. Stevia, a natural sweetener with medicinal properties and also having nutritional, therapeutic and industrial importance is being used all over the world. Stevia rebaudiana leaves are usually referred to as candy, sweet and honey leaves. Diterpene glycosides are responsible for its high sweetening potential of leaves. The phytochemical properties of bioactive chemicals present in stevia leaves are involves in maintaining the physiological functions of human body. Paper also highlights the importance of nutritional aspects of dried stevia leaves, metabolism of stevia, effects of it consumption on human health and clinical studies related to stevia ingestion. Various medicinal properties of stevia leaves discussed in paper like anti-hyperglycemia, anti-oxidative, hypotensive, nephro-protective, hepato protective, antibacterial and antifungal. Basic purpose of this review to understand the medicinalKeywords: potential Stevia; Diabetes;of stevia and Phytochemicals; its acceptance Medicinal as a significant plant; Steviol;raw material Nutrition; for human Disorders diet. -
Show Activity
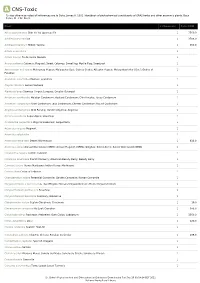
A CNS-Toxic *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Abies sachalinensis Shin-Yo-Yu; Japanese Fir 1 7560.0 Achillea moschata Iva 1 3708.0 Achillea millefolium Milfoil; Yarrow 1 550.0 Acinos suaveolens 1 Acinos alpinus Te de Sierra Nevada 1 Acorus calamus Calamus; Flagroot; Sweet Calamus; Sweetflag; Myrtle Flag; Sweetroot 1 Aframomum melegueta Melegueta Pepper; Malagueta (Sp.); Guinea Grains; Alligator Pepper; Malagettapfeffer (Ger.); Grains-of- 1 Paradise Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 Amomum xanthioides Malabar Cardamom; Bastard Cardamom; Chin Kousha; Tavoy Cardamom 1 Amomum compactum Siam Cardamom; Java Cardamom; Chester Cardamom; Round Cardamom 1 Angelica archangelica Wild Parsnip; Garden Angelica; Angelica 1 Annona squamosa Sugar-Apple; Sweetsop 1 Aristolochia serpentaria Virginia Snakeroot; Serpentaria 1 Artemisia vulgaris Mugwort 1 Artemisia salsoloides 1 Artemisia herba-alba Desert Wormwood 1 638.0 Artemisia annua Annual Wormwood (GRIN); Annual Mugwort (GRIN); Qinghao; Sweet Annie; Sweet Wormwood (GRIN) 1 Calamintha nepeta Turkish Calamint 1 Callicarpa americana French Mulberry; American Beauty Berry; Beauty Berry 1 Cannabis sativa Hemp; Marijuana; Indian Hemp; Marihuana 1 Cedrus libani Cedar of Lebanon 1 Chamaemelum nobile Perennial Camomile; Garden Camomile; Roman Camomile -

In Chemistry, Glycosides Are Certain Molecules in Which a Sugar Part Is
GLYCOSIDES Glycosides may be defined as the organic compounds from plants or animal sources, which on enzymatic or acid hydrolysis give one or more sugar moieties along with non- sugar moiety. Glycosides play numerous important roles in living organisms. Many plants store important chemicals in the form of inactive glycosides; if these chemicals are needed, the glycosides are brought in contact with water and an enzyme, and the sugar part is broken off, making the chemical available for use. Many such plant glycosides are used as medications. In animals (including humans), poisons are often bound to sugar molecules in order to remove them from the body. Formally, a glycoside is any molecule in which a sugar group is bonded through its carbon atom to another group via an O-glycosidic bond or an S-glycosidic bond; glycosides involving the latter are also called thioglycosides. The sugar group is then known as the glycone and the non-sugar group as the aglycone or genin part of the glycoside. The glycone can consist of a single sugar group (monosaccharide) or several sugar groups (oligosaccharide). Classification Classification based on linkages Based on the linkage of sugar moiety to aglycone part 1. O-Glycoside:-Here the sugar is combined with alcoholic or phenolic hydroxyl function of aglycone.eg:-digitalis. 2. N-glycosides:-Here nitrogen of amino group is condensed with a sugar ,eg- Nucleoside 3. S-glycoside:-Here sugar is combined with sulphur of aglycone,eg- isothiocyanate glycosides. 4. C-glycosides:-By condensation of a sugar with a cabon atom, eg-Cascaroside, aloin. Glycosides can be classified by the glycone, by the type of glycosidic bond, and by the aglycone. -

Herbs Are Obtained from the Leaves of Herbaceous (Non-Woody) Plants
SPICE UP YOUR DIET -- AND YOUR HEALTH Spices not only taste good, but they're good for your health, as registered dietitian Keri Glassman explains: Calorie free medicine! That is the beauty of spices. Most people think of spices as ingredients to simply add flavor to meals without adding calories. They actually help you save calories by adding flavor and helping you avoid adding heavy sauces, butter or other fats... But the BEST part is that not only do they save you calories and add flavor, they are actually VERY high in nutritional value! Did you know that cinnamon has more antioxidants than blueberries? From helping you keep your mind young to controlling blood sugar, everyone should be adding some spice in their life! Herbs are obtained from the leaves of herbaceous (non-woody) plants. They are used for savory purposes in cooking and some have medicinal value. Herbs often are used in larger amounts than spices. Spices are obtained from roots, flowers, fruits, seeds or bark. Spices are native to warm tropical climates and can be woody or herbaceous plants. Spices often are more potent and stronger flavored than herbs; as a result they typically are used in smaller amounts. Some spices are used not only to add taste, but also as a preservative. Herbs and Spices: Herbs and spices are a great source of antioxidants, vitamin, and minerals. Basil: Source of vitamin K, iron, calcium, vitamin A, dietary fiber, manganese, magnesium, vitamin C and potassium. • Heart Protection- Basil is a good source of Vitamin A, a strong antioxidant that has been shown to protect against free radical damage that leads to build up of plaque on artery walls. -

Sharp's at Waterford Farm Your Neighborhood Farm Ask Us How To
Lemongrass – Essential for Thai Sharp’s at Waterford Herbs List cooking Farm Anise - Hyssop Lovage (Levistcum officinale) Farming in Howard County Basil Marjoram (Origanum majorana) since 1903 African Blue Amethyst Improved Purple Sweet Eleonora Zaatar, a hint of thyme, oregano & 4003 Jennings Chapel Rd. Elidia - Compact; container basil marjoram Brookeville, MD 20833 Genovese Golden - ornamental mostly Holy - Sacred Red and Green Tel: (410) 489-2572 Mint (Mentha sp.) Italian Large Leaf Chocolate Peppermint Lemon – Mrs. Burns www.sharpfarm.com Lemon Mint Mountain Mint Lettuce Leaf – Napoletano email: Peppermint Pineapple Mint Lime [email protected] Spearmint Sweet Thai Dark Opal Oregano (Origanum sp.) Red Rubin Greek Rutgers Devotion Zaatar ( a hint of thyme, oregano, & marjoram) Oreganum Syriaca) Borage: the herb of gladness Hot and Spicy - real tang, our favorite for adding to beans Catnip (Nepeta)- feline friends treat Parsley (Petroselinum crispum) Calendula, Neon Plain leaf (Italian or flat) Curly – double or triple Chamomile (German) Organic curled parsley (Bodegold) Italian Dark Green – Giant of Italy – huge leaves Your Neighborhood Chervil (Anthricus cerefolium) ‘crispum’ Vertissimo Farm Rosemary (Rosmarinus) Arp Chives (Allium) Hill Hardy Med Leaf (Purly) Ask Us How to Garden Salem Large leaf (staro) Sage (Salvia offincinalis) Helpful Hints: We pride ourselves Cilantro (Coriandrum sativium) Garden - Extrakta on knowing how to vegetable and herb Cruiser – more upright – great for Pineapple garden. Please ask if you need bunching – 50 days Savory Winter information on how to. Yields? Cutting Celery (Apium graveolens) Sorrel, French Spacing between plants? Staking? aka leaf celery When you plant, space your harvest Stevia (Stevia rebaudiana) by using varieties of different maturity Dill (Anethum graveolens): Nature’s natural sweetener dates.