Ciz1 Promotes Mammalian DNA Replication
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Akt1 and Dciz1 Promote Cell Survival from Apoptotic Caspase Activation
ARTICLE https://doi.org/10.1038/s41467-020-19068-2 OPEN Akt1 and dCIZ1 promote cell survival from apoptotic caspase activation during regeneration and oncogenic overgrowth ✉ ✉ Gongping Sun1,2 , Xun Austin Ding2, Yewubdar Argaw2, Xiaoran Guo 2 & Denise J. Montell 2 Apoptosis is an ancient and evolutionarily conserved cell suicide program. During apoptosis, executioner caspase enzyme activation has been considered a point of no return. However, 1234567890():,; emerging evidence suggests that some cells can survive caspase activation following expo- sure to apoptosis-inducing stresses, raising questions as to the physiological significance and underlying molecular mechanisms of this unexpected phenomenon. Here, we show that, following severe tissue injury, Drosophila wing disc cells that survive executioner caspase activation contribute to tissue regeneration. Through RNAi screening, we identify akt1 and a previously uncharacterized Drosophila gene CG8108, which is homologous to the human gene CIZ1, as essential for survival from the executioner caspase activation. We also show that cells expressing activated oncogenes experience apoptotic caspase activation, and that Akt1 and dCIZ1 are required for their survival and overgrowth. Thus, survival following executioner caspase activation is a normal tissue repair mechanism usurped to promote oncogene-driven overgrowth. 1 The Key Laboratory of Experimental Teratology, Ministry of Education and Department of Anatomy and Histoembryology, School of Basic Medical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China. 2 Molecular, Cellular, and Developmental Biology Department, University of ✉ California, Santa Barbara, CA 93106, USA. email: [email protected]; [email protected] NATURE COMMUNICATIONS | (2020) 11:5726 | https://doi.org/10.1038/s41467-020-19068-2 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-020-19068-2 recise control of cell death and survival is critical for tissue we took advantage of the CasExpress biosensor11. -

Maintenance of Epigenetic Landscape Requires CIZ1 and Is Corrupted in Differentiated fibroblasts in Long-Term Culture
ARTICLE https://doi.org/10.1038/s41467-018-08072-2 OPEN Maintenance of epigenetic landscape requires CIZ1 and is corrupted in differentiated fibroblasts in long-term culture Emma R. Stewart1, Robert M.L. Turner1, Katherine Newling 2, Rebeca Ridings-Figueroa1,3, Victoria Scott1, Peter D. Ashton2, Justin F.X. Ainscough 1 & Dawn Coverley1 1234567890():,; The inactive X chromosome (Xi) serves as a model for establishment and maintenance of repressed chromatin and the function of polycomb repressive complexes (PRC1/2). Here we show that Xi transiently relocates from the nuclear periphery towards the interior during its replication, in a process dependent on CIZ1. Compromised relocation of Xi in CIZ1-null primary mouse embryonic fibroblasts is accompanied by loss of PRC-mediated H2AK119Ub1 and H3K27me3, increased solubility of PRC2 catalytic subunit EZH2, and genome-wide deregulation of polycomb-regulated genes. Xi position in S phase is also corrupted in cells adapted to long-term culture (WT or CIZ1-null), and also accompanied by specific changes in EZH2 and its targets. The data are consistent with the idea that chromatin relocation during S phase contributes to maintenance of epigenetic landscape in primary cells, and that elevated soluble EZH2 is part of an error-prone mechanism by which modifying enzyme meets template when chromatin relocation is compromised. 1 Department of Biology, University of York, York YO10 5DD, UK. 2 York Bioscience Technology Facility, University of York, York YO10 5DD, UK. 3Present address: Department of Genetics, University of Cambridge, Cambridge CB2 3EH, UK. Correspondence and requests for materials should be addressed to D.C. (email: [email protected]) NATURE COMMUNICATIONS | (2019) 10:460 | https://doi.org/10.1038/s41467-018-08072-2 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-018-08072-2 he inactive X chromosome (Xi) is a discrete unit of attachment at Xi is by association with RNA5, most likely Xist6,17. -

Identification of DHX9 As a Cell Cycle Regulated Nucleolar Recruitment
www.nature.com/scientificreports OPEN Identifcation of DHX9 as a cell cycle regulated nucleolar recruitment factor for CIZ1 Urvi Thacker1, Tekle Pauzaite1, James Tollitt1, Maria Twardowska1, Charlotte Harrison2, Adam Dowle3, Dawn Coverley2 & Nikki A. Copeland1* CIP1-interacting zinc fnger protein 1 (CIZ1) is a nuclear matrix associated protein that facilitates a number of nuclear functions including initiation of DNA replication, epigenetic maintenance and associates with the inactive X-chromosome. Here, to gain more insight into the protein networks that underpin this diverse functionality, molecular panning and mass spectrometry are used to identify protein interaction partners of CIZ1, and CIZ1 replication domain (CIZ1-RD). STRING analysis of CIZ1 interaction partners identifed 2 functional clusters: ribosomal subunits and nucleolar proteins including the DEAD box helicases, DHX9, DDX5 and DDX17. DHX9 shares common functions with CIZ1, including interaction with XIST long-non-coding RNA, epigenetic maintenance and regulation of DNA replication. Functional characterisation of the CIZ1-DHX9 complex showed that CIZ1-DHX9 interact in vitro and dynamically colocalise within the nucleolus from early to mid S-phase. CIZ1-DHX9 nucleolar colocalisation is dependent upon RNA polymerase I activity and is abolished by depletion of DHX9. In addition, depletion of DHX9 reduced cell cycle progression from G1 to S-phase in mouse fbroblasts. The data suggest that DHX9-CIZ1 are required for efcient cell cycle progression at the G1/S transition and that nucleolar recruitment is integral to their mechanism of action. Te precise duplication of the genome is a highly regulated process that ensures genomic stability. Te forma- tion of putative replication origins, origin licensing and initiation of DNA replication are highly orchestrated processes that are tightly regulated by sequential cyclin-CDK complexes and Dbf4 dependent kinase (DDK)1,2. -

Supplementary Materials
Supplementary materials Supplementary Table S1: MGNC compound library Ingredien Molecule Caco- Mol ID MW AlogP OB (%) BBB DL FASA- HL t Name Name 2 shengdi MOL012254 campesterol 400.8 7.63 37.58 1.34 0.98 0.7 0.21 20.2 shengdi MOL000519 coniferin 314.4 3.16 31.11 0.42 -0.2 0.3 0.27 74.6 beta- shengdi MOL000359 414.8 8.08 36.91 1.32 0.99 0.8 0.23 20.2 sitosterol pachymic shengdi MOL000289 528.9 6.54 33.63 0.1 -0.6 0.8 0 9.27 acid Poricoic acid shengdi MOL000291 484.7 5.64 30.52 -0.08 -0.9 0.8 0 8.67 B Chrysanthem shengdi MOL004492 585 8.24 38.72 0.51 -1 0.6 0.3 17.5 axanthin 20- shengdi MOL011455 Hexadecano 418.6 1.91 32.7 -0.24 -0.4 0.7 0.29 104 ylingenol huanglian MOL001454 berberine 336.4 3.45 36.86 1.24 0.57 0.8 0.19 6.57 huanglian MOL013352 Obacunone 454.6 2.68 43.29 0.01 -0.4 0.8 0.31 -13 huanglian MOL002894 berberrubine 322.4 3.2 35.74 1.07 0.17 0.7 0.24 6.46 huanglian MOL002897 epiberberine 336.4 3.45 43.09 1.17 0.4 0.8 0.19 6.1 huanglian MOL002903 (R)-Canadine 339.4 3.4 55.37 1.04 0.57 0.8 0.2 6.41 huanglian MOL002904 Berlambine 351.4 2.49 36.68 0.97 0.17 0.8 0.28 7.33 Corchorosid huanglian MOL002907 404.6 1.34 105 -0.91 -1.3 0.8 0.29 6.68 e A_qt Magnogrand huanglian MOL000622 266.4 1.18 63.71 0.02 -0.2 0.2 0.3 3.17 iolide huanglian MOL000762 Palmidin A 510.5 4.52 35.36 -0.38 -1.5 0.7 0.39 33.2 huanglian MOL000785 palmatine 352.4 3.65 64.6 1.33 0.37 0.7 0.13 2.25 huanglian MOL000098 quercetin 302.3 1.5 46.43 0.05 -0.8 0.3 0.38 14.4 huanglian MOL001458 coptisine 320.3 3.25 30.67 1.21 0.32 0.9 0.26 9.33 huanglian MOL002668 Worenine -

Exploring the Role of Polycomb Recruitment in Xist-Mediated Silencing of the X Chromosome in ES Cells
bioRxiv preprint doi: https://doi.org/10.1101/495739; this version posted December 13, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Exploring the role of Polycomb recruitment in Xist-mediated silencing of the X chromosome in ES cells Aurélie Bousard1*, Ana Cláudia Raposo2*, Jan Jakub Żylicz1,3*, Christel Picard1, Vanessa Borges Pires2,4, Yanyan Qi5, Laurène Syx1, Howard Y. Chang5,6, Edith Heard1†, Simão Teixeira da Rocha1,2† 1 Mammalian Developmental Epigenetics Group, Institut Curie, PSL University, CNRS UMR3215, INSERM U934, 26, rue d'Ulm, 75005 Paris, France. 2 Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, 1649- 028 Lisboa, Portugal. 3 University of Cambridge, Department of Physiology, Development and Neuroscience, Downing Street, Cambridge CB2 3EG, UK. 4 Instituto de Ciências Biomédicas Abel Salazar, Universidade do Porto, 4050-313 Porto, Portugal. 5 Center for Dynamic Personal Regulomes, Stanford University, Stanford, CA 94305, USA. 6 Howard Hughes Medical Institute, Stanford University, Stanford, CA 94305, USA. * these authors contributed equally † co-corresponding authors Correspondence: Edith Heard: [email protected] Simão Teixeira da Rocha: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/495739; this version posted December 13, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Bousard, Raposo, Zylicz et al., Abstract Xist RNA has been established as the master regulator of X-chromosome inactivation (XCI) in female eutherian mammals but its mechanism of action remains unclear. -

The Nuclear Matrix Protein CIZ1 Facilitates Localization of Xist RNA to the Inactive X-Chromosome Territory
Downloaded from genesdev.cshlp.org on October 3, 2021 - Published by Cold Spring Harbor Laboratory Press The nuclear matrix protein CIZ1 facilitates localization of Xist RNA to the inactive X-chromosome territory Rebeca Ridings-Figueroa,1,7 Emma R. Stewart,1 Tatyana B. Nesterova,2 Heather Coker,2 Greta Pintacuda,2 Jonathan Godwin,2 Rose Wilson,1,8 Aidan Haslam,1 Fred Lilley,1 Renate Ruigrok,3 Sumia A. Bageghni,3 Ghadeer Albadrani,3,4 William Mansfield,5 Jo-An Roulson,6 Neil Brockdorff,2 Justin F.X. Ainscough,1,3 and Dawn Coverley1 1Department of Biology, University of York, York YO10 5DD, United Kingdom; 2Department of Biochemistry, University of Oxford, Oxford OX1 3QU, United Kingdom; 3Leeds Institute of Cardiovascular and Metabolic Medicine (LICAMM), University of Leeds, Leeds LS2 9JT, United Kingdom; 4Princess Nourah Bint Abdulrahman University (PNU), Riyadh, Kingdom of Saudi Arabia; 5Stem Cell Institute, University of Cambridge, Cambridge CB2 1QR, United Kingdom; 6Leeds Institute of Molecular Medicine (LIMM), University of Leeds, Leeds LS9 7TF, United Kingdom The nuclear matrix protein Cip1-interacting zinc finger protein 1 (CIZ1) promotes DNA replication in association with cyclins and has been linked to adult and pediatric cancers. Here we show that CIZ1 is highly enriched on the inactive X chromosome (Xi) in mouse and human female cells and is retained by interaction with the RNA-de- pendent nuclear matrix. CIZ1 is recruited to Xi in response to expression of X inactive-specific transcript (Xist) RNA during the earliest stages of X inactivation in embryonic stem cells and is dependent on the C-terminal nuclear matrix anchor domain of CIZ1 and the E repeats of Xist. -

A Polyglutamine Domain Is Required for De Novo CIZ1 Assembly Formation at the Inactive X Chromosome
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.10.376558; this version posted November 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. CIZ1 assembly at the inactive X-chromosome A polyglutamine domain is required for de novo CIZ1 assembly formation at the inactive X chromosome Sajad Sofi1,2, Louisa Williamson1,2, Gabrielle L. Turvey1,2, Charlotte Scoynes1,3, Claire Hirst1, Jonathan Godwin4, Neil Brockdorff4, Justin Ainscough1, 2, Dawn Coverley1, 2* 1 Department of Biology, University of York, York, YO10 5DD, United Kingdom 2 York Biomedical Research Institute, University of York, York, YO10 5DD, United Kingdom 3 College of Science and Engineering, University of Edinburgh, Edinburgh, EH1 2QL, United Kingdom 4 Department of Biochemistry, University of Oxford, Oxford, OX1 3QU, United Kingdom *Correspondence: Dawn Coverley ([email protected]) 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.10.376558; this version posted November 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. CIZ1 assembly at the inactive X-chromosome Summary CIP1-interacting zinc finger protein 1 (CIZ1) forms large assemblies at the inactive X chromosome (Xi) in female fibroblasts in an Xist lncRNA-dependent manner. -

Identification and Analysis of the Signalling Networks That Regulate Ciz1 Levels in Normal and Cancer Cell Lines
Identification and analysis of the signalling networks that regulate Ciz1 levels in normal and cancer cell lines PhD Biomedical and Life Sciences By Teklė Paužaitė Lancaster University A Thesis submitted in fulfilment of requirement for the degree of Doctor of Philosophy March 2019 Declaration This dissertation is the result of my own work and includes nothing, which is the outcome of work done in collaboration except where specifically indicated in the text. It has not been previously submitted, in part or whole, to any university of institution for any degree, diploma, or other qualification. Signed: Teklė Paužaitė Date: 01 07 2019 II Dedication This thesis is dedicated to the strongest, most caring and extraordinary person I know, my mother Eglė Paužienė. III Abstract Ciz1 is a nuclear protein that associates with cyclin A – cyclin dependent kinase 2 (CDK2) and facilitates the initiation of DNA replication. Ciz1 overexpression has been linked to common cancer types, including breast, colon, prostate, lung, and liver cancers. This suggests that identification of mechanisms that regulate Ciz1 levels may represent potential drug targets in cancer. This work identifies that CDK2 and DDK activity are required to maintain Ciz1 levels. Chemical or genetic inhibition of CDK2 or DDK (Cdc7-Dbf4) activity in murine fibroblasts reduced Ciz1 levels. Further analysis demonstrated that CDK and DDK activity promotes Ciz1 accumulation in G1 phase by reducing ubiquitin proteasome system (UPS) mediated degradation. Furthermore, Ciz1 levels are actively controlled by the proteasome, as inhibition of protein translation rapidly reduced Ciz1 levels, and this is reversed by proteasomal inhibition. The data suggest a model where Ciz1 is regulated by opposing kinase and UPS activities, leading to Ciz1 accumulation in response to rising kinase activity in G1 phase, and its degradation later in the cell cycle. -

NCBI2R - to Navigate and Annotate Genes and Snps
NCBI2R - To navigate and annotate genes and SNPs. The Problem • Genome Wide Analysis provides lots of p- values but without full context The Solution • Annotation Scott Melville ([email protected]) Why use NCBI 2 R? • Simple to use • No mirrors or out of date information • Uses NCBI. Scott Melville ([email protected]) GWA results – with SNP names, p-values, effect sizes etc Position, Gene? Fxn_class, GetSNPInfo NCBI Locus ID numbers Pathways, phenotypes, position, orientation, GetGeneInfo OMIM links, summaries, interacting genes GetNeighbours(genetic positions) List of neighbouring genes within a user-specified distance (eg 100K). Creates links. MakeHTML Scott Melville ([email protected]) How To Analyse Results marker p-values n beta rs532523 0.015512 286 -0.5252 rs696786 0.000012 293 0.2142 rs626346 0.4240505 283 0.0622 > snplist<-anydf$marker > snplist<-table[order(table$p),][1:100,"marker"] > GetSNPInfo(snplist) Scott Melville ([email protected]) GetSNPInfo > GetSNPInfo(“rs12456”) marker genesymbol locusID chr chrpos fxn_class rs12334 CIZ1 25792 9 129979750 missense > GetSNPInfo(c("rs12456","rs626616")) marker genesymbol locusID chr chrpos fxn_class rs12334 CIZ1 25792 9 129979750 missense rs626616 19 60723974 Scott Melville ([email protected]) showurl=TRUE > GetSNPInfo(“rs12456”,showurl=T) http://eutils.ncbi.nlm.nih.gov/entrez/eutils/ efetch.fcgi?db=snp&id=12356 &report=DocSet &tool=NCBI2R&[email protected] Scott Melville ([email protected]) GWA results – with SNP names, p-values, effect sizes etc Position, Gene? Fxn_class, GetSNPInfo NCBI Locus ID numbers Pathways, phenotypes, position, orientation, GetGeneInfo OMIM links, summaries, interacting genes GetNeighbours(genetic positions) List of neighbouring genes within a user-specified distance (eg 100K). -

Identification of Novel Cerebellar Developmental Transcriptional Regulators with Motif Activity Analysis Thomas J
Ha et al. BMC Genomics (2019) 20:718 https://doi.org/10.1186/s12864-019-6063-9 RESEARCH ARTICLE Open Access Identification of novel cerebellar developmental transcriptional regulators with motif activity analysis Thomas J. Ha1,8†, Peter G. Y. Zhang1†, Remi Robert1, Joanna Yeung1, Douglas J. Swanson1, Anthony Mathelier1,2,3, Wyeth W. Wasserman1, Sujin Im1, Masayoshi Itoh4,5, Hideya Kawaji4,5, Timo Lassmann4,5,7, Carsten O. Daub4,5, Erik Arner4,5, The FANTOM Consortium4,5, Piero Carninci4,5, Yoshihide Hayashizaki4,6, Alistair R. R. Forrest4,5 and Daniel Goldowitz1* Abstract Background: The work of the FANTOM5 Consortium has brought forth a new level of understanding of the regulation of gene transcription and the cellular processes involved in creating diversity of cell types. In this study, we extended the analysis of the FANTOM5 Cap Analysis of Gene Expression (CAGE) transcriptome data to focus on understanding the genetic regulators involved in mouse cerebellar development. Results: We used the HeliScopeCAGE library sequencing on cerebellar samples over 8 embryonic and 4 early postnatal times. This study showcases temporal expression pattern changes during cerebellar development. Through a bioinformatics analysis that focused on transcription factors, their promoters and binding sites, we identified genes that appear as strong candidates for involvement in cerebellar development. We selected several candidate transcriptional regulators for validation experiments including qRT-PCR and shRNA transcript knockdown. We observed marked and reproducible developmental defects in Atf4, Rfx3, and Scrt2 knockdown embryos, which support the role of these genes in cerebellar development. Conclusions: The successful identification of these novel gene regulators in cerebellar development demonstrates that the FANTOM5 cerebellum time series is a high-quality transcriptome database for functional investigation of gene regulatory networks in cerebellar development. -
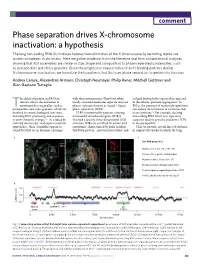
Phase Separation Drives X-Chromosome Inactivation
comment Phase separation drives X-chromosome inactivation: a hypothesis The long non-coding RNA Xist induces heterochromatinization of the X chromosome by recruiting repressive protein complexes to chromatin. Here we gather evidence, from the literature and from computational analyses, showing that Xist assemblies are similar in size, shape and composition to phase-separated condensates, such as paraspeckles and stress granules. Given the progressive sequestration of Xist’s binding partners during X-chromosome inactivation, we formulate the hypothesis that Xist uses phase separation to perform its function. Andrea Cerase, Alexandros Armaos, Christoph Neumayer, Philip Avner, Mitchell Guttman and Gian Gaetano Tartaglia he ability of protein and RNA to with their environment. They form when isolated hydrophobic regions that, exposed interact affects the formation of locally saturated molecules separate into two to the solvent, promote aggregation9. In membrane-less organelles, such as phases, a process known as ‘liquid–liquid RNAs, the presence of nucleotide repetitions T 7 paraspeckles and stress granules, which are phase separation’ (LLPS) . can induce the formation of structures that involved in various biological functions1, LLPS is promoted by proteins carrying attract proteins2,4. For example, the long including RNA processing and responses intrinsically disordered regions (IDRs) non-coding RNA Neat1 uses repeats to to environmental changes2–4. As studied by that lack a specific three-dimensional (3D) sequester specific proteins and -

Supporting Information
Supporting Information Higgins et al. 10.1073/pnas.1210107109 SI Results mL. In vivo analysis was carried out at Southern Research In- At the time of analysis more than 600 ESTs derived from normal stitute using 6-wk-old female NOD/SCID mice from the Na- and diseased tissues and cell lines mapped to the National Center tional Cancer Institute National Laboratory for Cancer for Biotechnology Information unigene cluster Hs. 212395 for Research. Forty mice were assigned randomly to three groups on human Ciz1 (www.ncbi.nlm.nih.gov/sites/entrez?db=unigene). In- day −3. On day −3, 2% sucrose was added to the drinking water dividual ESTs were translated, and the predicted amino acid se- of groups 1 and 2 (15 mice each), and 2 mg/mL doxycycline in quences were compared with full-length Ciz1 with the aim of 2% sucrose was added to the water of group 3 (10 mice). On day 7 verifying the existence of b-variant Ciz1. Ciz1 transcripts that span 0, mice were inoculated s.c. on the right flank with 1.0 × 10 exons 14 and 15 were detected in a total of 39 EST libraries, in- SBC5 ptTS-Neo b-variant shRNA 4 cells (90–95% confluence, cluding 28 that were derived from tumors. Three of five libraries 98.7% viability), in 0.1 mL medium without G418 and hygrom- derived from neuroendocrine lung cancers, 4 of 22 carcinoma li- ycin. On day 21, mice in group 2 were switched to water containing braries, 0 of 3 normal lung libraries, and 1 of 9 libraries derived sucrose and doxycycline.