Selection of Native Tree Species for Subtropical Forest Restoration in Southwest China
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spatial Distribution and Historical Dynamics of Threatened Conifers of the Dalat Plateau, Vietnam
SPATIAL DISTRIBUTION AND HISTORICAL DYNAMICS OF THREATENED CONIFERS OF THE DALAT PLATEAU, VIETNAM A thesis Presented to The Faculty of the Graduate School At the University of Missouri In Partial Fulfillment Of the Requirements for the Degree Master of Arts By TRANG THI THU TRAN Dr. C. Mark Cowell, Thesis Supervisor MAY 2011 The undersigned, appointed by the dean of the Graduate School, have examined the thesis entitled SPATIAL DISTRIBUTION AND HISTORICAL DYNAMICS OF THREATENED CONIFERS OF THE DALAT PLATEAU, VIETNAM Presented by Trang Thi Thu Tran A candidate for the degree of Master of Arts of Geography And hereby certify that, in their opinion, it is worthy of acceptance. Professor C. Mark Cowell Professor Cuizhen (Susan) Wang Professor Mark Morgan ACKNOWLEDGEMENTS This research project would not have been possible without the support of many people. The author wishes to express gratitude to her supervisor, Prof. Dr. Mark Cowell who was abundantly helpful and offered invaluable assistance, support, and guidance. My heartfelt thanks also go to the members of supervisory committees, Assoc. Prof. Dr. Cuizhen (Susan) Wang and Prof. Mark Morgan without their knowledge and assistance this study would not have been successful. I also wish to thank the staff of the Vietnam Initiatives Group, particularly to Prof. Joseph Hobbs, Prof. Jerry Nelson, and Sang S. Kim for their encouragement and support through the duration of my studies. I also extend thanks to the Conservation Leadership Programme (aka BP Conservation Programme) and Rufford Small Grands for their financial support for the field work. Deepest gratitude is also due to Sub-Institute of Ecology Resources and Environmental Studies (SIERES) of the Institute of Tropical Biology (ITB) Vietnam, particularly to Prof. -

Distribution and Conservation Status of Shortridge's Capped Langurs
Distribution and conservation status of Shortridge’s capped langurs Trachypithecus shortridgei in China L IANG-WEI C UI,YING-CHUN L I ,CHI M A ,MATTHEW B. SCOTT,JIN-FA L I X IAO-YANG H E ,DONG-HUI L I ,JUN S UN,WEN-MO S UN and W EN X IAO Abstract We conducted community interviews and field and in south-western China in the Nu and Dulong valleys surveys to determine the distribution and population of (Pocock, ; Groves, ; Htun et al., ). Numbers the Endangered Shortridge’s capped langur Trachypithecus of individuals are assumed to be low and declining as a result shortridgei, and the threats to the species, in the Dulong and of a restricted geographical range, hunting pressure and Nu River valleys of north-western Yunnan Province, China. widespread deforestation for agriculture and timber extrac- We found that c. groups of T. shortridgei reside in the tion. The total population is believed to have declined by at Dulong valley, mostly located in the southern portion of least % since , primarily as a result of hunting and the valley. According to interview and observational records habitat loss (Htun et al., ). Consequently, T. shortridgei in the Gaoligong Mountains to the west of the Nu River, is categorized as Endangered on the IUCN Red List (Htun individuals and no groups were observed. Family groups et al., ) and is listed in CITES Appendix I (CITES, consist of one adult male, – adult females and up to five ). In China it is a Category I protected species under young. We estimate the population of T. -

Two New Species of the Orb-Weaver Genus Chorizopes from Yunnan
A peer-reviewed open-access journal ZooKeys 626: 45–55Two (2016) new species of the orb-weaver genus Chorizopes from Yunnan, China... 45 doi: 10.3897/zookeys.626.7485 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Two new species of the orb-weaver genus Chorizopes from Yunnan, China (Araneae, Araneidae) Xiao-Qi Mi1, Cheng Wang1, Xian-Jin Peng2 1 College of Biological, Agricultural and Forest Engineering, Tongren University, Tongren, Guizhou 554300, China 2 College of Life Sciences, Hunan Normal University, Changsha, Hunan 410081, China Corresponding author: Xian-Jin Peng ([email protected]) Academic editor: S. Li | Received 11 December 2015 | Accepted 6 October 2016 | Published 20 October 2016 http://zoobank.org/AC23216F-E284-4344-9890-A6976C0FFFF5 Citation: Mi X-Q, Wang C, Peng X-J (2016) Two new species of the orb-weaver genus Chorizopes from Yunnan, China (Araneae, Araneidae). ZooKeys 626: 45–55. doi: 10.3897/zookeys.626.7485 Abstract Two new species of the orb-weaver genus Chorizopes from Yunnan Province, China are described: C. albus sp. n. (male and female) from the Gaoligong Mountains and Ailao Mountains, and C. longus sp. n. (male and female) from the Gaoligong Mountains. Chorizopes albus sp. n. can be distinguished from the related species C. shimenensis by: 1) median apophysis widest at the middle part versus widest at the base in the latter; 2) median apophysis without the dorsal spur found in that of the latter; 3) spermathecae spherical versus ovoid in the latter; 4) having one pair of large white spots on posterior lateral area of abdomen versus having two pairs of crescent white patches with dark edges on dorsal abdomen in the latter. -

Assessing Restoration Potential of Fragmented and Degraded Fagaceae Forests in Meghalaya, North-East India
Article Assessing Restoration Potential of Fragmented and Degraded Fagaceae Forests in Meghalaya, North-East India Prem Prakash Singh 1,2,* , Tamalika Chakraborty 3, Anna Dermann 4 , Florian Dermann 4, Dibyendu Adhikari 1, Purna B. Gurung 1, Saroj Kanta Barik 1,2, Jürgen Bauhus 4 , Fabian Ewald Fassnacht 5, Daniel C. Dey 6, Christine Rösch 7 and Somidh Saha 4,7,* 1 Department of Botany, North-Eastern Hill University, Shillong 793022, India; [email protected] (D.A.); [email protected] (P.B.G.); [email protected] (S.K.B.) 2 CSIR-National Botanical Research Institute, Council of Scientific & Industrial Research, Rana Pratap Marg, Lucknow 226001, Uttar Pradesh, India 3 Institute of Forest Ecosystems, Thünen Institute, Alfred-Möller-Str. 1, House number 41/42, D-16225 Eberswalde, Germany; [email protected] 4 Chair of Silviculture, University of Freiburg, Tennenbacherstr. 4, D-79085 Freiburg, Germany; anna-fl[email protected] (A.D.); fl[email protected] (F.D.); [email protected] (J.B.) 5 Institute for Geography and Geoecology, Karlsruhe Institute of Technology, Kaiserstr. 12, D-76131 Karlsruhe, Germany; [email protected] 6 Northern Research Station, USDA Forest Service, 202 Natural Resources Building, Columbia, MO 65211-7260, USA; [email protected] 7 Institute for Technology Assessment and Systems Analysis, Karlsruhe Institute of Technology, Karlstr. 11, D-76133 Karlsruhe, Germany; [email protected] * Correspondence: prem12fl[email protected] (P.P.S.); [email protected] (S.S.) Received: 5 August 2020; Accepted: 16 September 2020; Published: 19 September 2020 Abstract: The montane subtropical broad-leaved humid forests of Meghalaya (Northeast India) are highly diverse and situated at the transition zone between the Eastern Himalayas and Indo-Burma biodiversity hotspots. -

Butterfly Diversity in Human-Modified Ecosystems of Southern Sikkim, the Eastern Himalaya, India
OPEN ACCESS The Journal of Threatened Taxa is dedicated to building evidence for conservaton globally by publishing peer-reviewed artcles online every month at a reasonably rapid rate at www.threatenedtaxa.org. All artcles published in JoTT are registered under Creatve Commons Atributon 4.0 Internatonal License unless otherwise mentoned. JoTT allows unrestricted use of artcles in any medium, reproducton, and distributon by providing adequate credit to the authors and the source of publicaton. Journal of Threatened Taxa Building evidence for conservaton globally www.threatenedtaxa.org ISSN 0974-7907 (Online) | ISSN 0974-7893 (Print) Article Butterfly diversity in human-modified ecosystems of southern Sikkim, the eastern Himalaya, India Prem Kumar Chetri, Kishor Sharma, Sailendra Dewan & Bhoj Kumar Acharya 26 April 2018 | Vol. 10 | No. 5 | Pages: 11551-11565 10.11609/jot.3641.10.5.11551-11565 For Focus, Scope, Aims, Policies and Guidelines visit htp://threatenedtaxa.org/index.php/JoTT/about/editorialPolicies#custom-0 For Artcle Submission Guidelines visit htp://threatenedtaxa.org/index.php/JoTT/about/submissions#onlineSubmissions For Policies against Scientfc Misconduct visit htp://threatenedtaxa.org/index.php/JoTT/about/editorialPolicies#custom-2 For reprints contact <[email protected]> Publisher & Host Partners Member Threatened Taxa Journal of Threatened Taxa | www.threatenedtaxa.org | 26 April 2018 | 10(5): 11551–11565 Article Butterfly diversity in human-modified ecosystems of southern Sikkim, the eastern Himalaya, India Prem -

Yunnan Sustainable Road Maintenance (Sector) Project (RRP PRC 45030)
Yunnan Sustainable Road Maintenance (Sector) Project (RRP PRC 45030) Initial Environmental Examination April 2013 PRC: Yunnan Sustainable Road Maintenance (Sector) Project Prepared by the Yunnan Highway Administration Bureau for the Asian Development Bank CURRENCY EQUIVALENTS (as of 23 April 2013) Currency unit – Yuan (CNY) CNY1.00 = $0.1616 $1.00 = CNY6.1871 ABBREVIATIONS ADB – Asian Development Bank ADB SPS – Asian Development Bank Safeguard Policy Statement 2009 CEWP – Construction Environmental Work Plan CIEE – Consolidated Initial Environmental Examination(i.e. many subprojects addressed in one IEE) dBA – A measure of audible (the ear) noise EARF – Environmental Assessment and Review Framework EIA – Environmental Impact Assessment EMP – Environmental Management Plan ESSU – Environment, Social and Safety Unit (established within YHAB) GRM – Grievance Redress Mechanism IEE – Initial Environmental Examination masl – metres above sea level PMO – Project Management Office PPTA – Project Preparation Technical Assistance PRC – People‟s Republic of China RP – Resettlement Plan RoW – Right of Way SDAP – Social Development Action Plan SPS 2009 – ADB‟s 2009 Safeguard Policy Statement subIDForm – Sub-identification form, provided in the project‟s Operations Manual summarizing all features for a subproject vpd – Vehicles per day YEPD – Yunnan Environmental Protection Department YHAB – Yunnan Highway Administration Bureau YHDIC – Yunnan Highway Development and Investment Co. Ltd YPDOT – Yunnan Provincial Department of Transportation YSRI – Yunnan Science and Technology Research Institute of Highways YTPRDI – Yunnan Transport Planning Research and Design Institute WEIGHTS AND MEASURES CO – Carbon monoxide kph – Kilometers per hour mu – Land measurement unit = 520m2 NO2 – Nitrate or Nitrogen Dioxide SO2 – Sulphur dioxide TPM – Suspended particulate matter, with particles ≥ 10 microns in size, and a danger to lungs. -
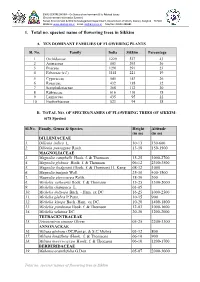
1. Total No. Species/ Name of Flowering Trees in Sikkim
ENVIS CENTRE SIKKIM – On Status of environment & Its Related Issues (Environmental Information System) Forest, Environment & Wildlife Management Department, Government of Sikkim, Deorali, Gangtok – 737102 Website: www.sikenvis.nic.in Email: [email protected] Tele/Fax: 03592-280381 1. Total no. species/ name of flowering trees in Sikkim A. TEN DOMINANT FAMILIES OF FLOWERING PLANTS Sl. No. Family India Sikkim Percentage 1 Orchidaceae 1229 527 43 2 Asteraceae 803 293 36 3 Poaceae 1291 291 23 4 Fabaceae (s.l.) 1141 221 19 5 Cyperaceae 545 143 26 6 Rosaceae 432 138 32 7 Scrophulariaceae 368 112 30 8 Rubiaceae 616 110 18 9 Lamiaceae 435 95 22 10 Euphorbiaceae 523 94 18 B. TOTAL NO. OF SPECIES/NAMES OF FLOWERING TREES OF SIKKIM: (678 Species) Sl.No. Family, Genus & Species Height Altitude (in m) (in m) DILLENIACEAE 1. Dillenia indica L. 10-13 150-600 2. Dillenia pentagyna Roxb. 13-18 150-1500 MAGNOLIACEAE 3. Magnolia campbelli Hook. f. & Thomson 15-25 1000-2700 4. Magnolia globosa Hook. f. & Thomson 06-12 2300-3500 5. Magnolia hodgsonii (Hook. f. & Thomson) H. Keng 08-12 1000 6. Magnolia insignis Wall. 25-30 400-1500 7. Magnolia pterocarpa Roxb. 18-30 500 8. Michelia cathcartii Hook. f. & Thomson 15-25 1500-2000 9. Michelia champaca L. 03-05 10. Michelia doltsopa Buch. - Ham. ex DC. 16-25 1000-2500 11. Michelia glabra P.Pann. 10-15 900 12. Michelia kisopa Buch.-Ham. ex DC. 10-20 1400-1800 13. Michelia punduana Hook. f. & Thomson 12-03 1000-1600 14. Michelia velutina DC. -

Post-Pliocene Establishment of the Present Monsoonal Climate in SW
EGU Journal Logos (RGB) Open Access Open Access Open Access Advances in Annales Nonlinear Processes Geosciences Geophysicae in Geophysics Open Access Open Access Natural Hazards Natural Hazards and Earth System and Earth System Sciences Sciences Discussions Open Access Open Access Atmospheric Atmospheric Chemistry Chemistry and Physics and Physics Discussions Open Access Open Access Atmospheric Atmospheric Measurement Measurement Techniques Techniques Discussions Open Access Open Access Biogeosciences Biogeosciences Discussions Open Access Open Access Clim. Past, 9, 1911–1920, 2013 Climate www.clim-past.net/9/1911/2013/ Climate doi:10.5194/cp-9-1911-2013 of the Past of the Past © Author(s) 2013. CC Attribution 3.0 License. Discussions Open Access Open Access Earth System Earth System Dynamics Dynamics Discussions Post-Pliocene establishment of the present monsoonal climate in SW Open Access Open Access China: evidence from the late Pliocene Longmen megafloraGeoscientific Geoscientific Instrumentation Instrumentation T. Su1,3, F. M. B. Jacques1, R. A. Spicer4,5, Y.-S. Liu6, Y.-J. Huang2, Y.-W. Xing7, and Z.-K.Methods Zhou1,2 and Methods and 1Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, ChineseData Academy Systems of Sciences, Data Systems Mengla 666303, China Discussions Open Access 2 Open Access Key Laboratory of Biodiversity and Biogeography, Kunming Institute of Botany, Chinese Academy of Sciences, Geoscientific Kunming 650204, China Geoscientific 3State Key Laboratory of Paleobiology and Stratigraphy, Nanjing Institute of Geology and Paleontology, Model Development Model Development the Chinese Academy of Sciences, Nanjing 210008, China Discussions 4Department of Earth and Environmental Sciences, Centre for Earth, Planetary, Space and Astronomical Research, The Open University, Milton Keynes, MK7 6AA, UK Open Access Open Access 5Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China Hydrology and Hydrology and 6Department of Biological Sciences, P.O. -

2015-5-263.Pdf
(4) Schmidtiphaea yunnanensis Davies et Yang, 1996 05.31, number 0059052–0059064 and 0059066 is 1992.05.24 Schmidtiphaea yunnanensis Davies et Yang, 1996 (Davies & on label (all of them are 1993.05.24/31 in original description). Yang, 1996). Type locality: Jiangcheng County, Yunnan Province, China. Holotype: number 0059002. Paratypes: 3 specimens, number 0059003–0059005 (number 0059003 was allotype in II. ISOPTERA original description). Type locality: Jiangcheng County, Yunnan Province, China. (i) Rhinotermitidae (11) Heterotermes coelceps Zhu, Huang et Wang, 1992 (ii) Gomphidae Heterotermes coelceps Zhu, Huang et Wang, 1992 (Zhu et al, (5) Anisogomphus nitidus Yang et Davies, 1993 1992). Anisogomphus nitidus Yang et Davies, 1993 (Yang & Davies, Syntypes: 23 specimens, number 0060275–0060297. 1993). Authors didn’t indicate the holotype. Holotype: number 0059006. Type locality: Qianjiang County, Chongqing City (Sichuan The collector of number 0059006 is Allen and Davies on label Province), China. (DALD in original description). (12) Heterotermes dayongensis Zhu, Huang et Wang, 1992 Type locality: Dali, Yunnan Province, China. Heterotermes dayongensis Zhu, Huang et Wang, 1992 (Zhu (6) Anisogomphus resortus Yang et Davies, 1996 et al, 1992). Anisogomphus resortus Yang et Davies, 1996 (Yang & Syntypes: 55 specimens, number 0060062–0060116. Davies, 1996). Authors didn’t indicate the holotype. Holotype: number 0059040. Paratype: 1 specimen, number Type locality: Zhangjiajie National Park, Dayong City, Hunan 0059041. Province, China. The collecting dates of number 0059040 and 0059041 are (13) Heterotermes leigongshanensis Zhu, Huang, Wang et 1993.06.10 and 1992.06.08 on label respectively (1993.06.08 Han, 1992 and 1992.07.10 in original description). Heterotermes leigongshanensis Zhu, Huang, Wang et Han, Type locality: Emeishan Mountain, Sichuan Province, China. -

Correlation Analysis of Compounds in Essential Oil of Amomum Tsaoko Seed and Fruit Morphological Characteristics, Geographical Conditions, Locality of Growth
agronomy Article Correlation Analysis of Compounds in Essential Oil of Amomum tsaoko Seed and Fruit Morphological Characteristics, Geographical Conditions, Locality of Growth Guodong Li 1,†, Qinwei Lu 2,† , Jingjian Wang 2, Qingyu Hu 2,3 , Pinghui Liu 2, Yaowen Yang 1, Yongkun Li 1,* , Huiru Tang 2,* and Hui Xie 2,* 1 Faculty of Chinese Pharmacy, Yunnan University of Traditional Chinese Medicine (TCM), No. 1076 Yuhua Road, Chenggong District, Kunming 650500, China; [email protected] (G.L.); [email protected] (Y.Y.) 2 Human Phenome Institute, Fudan University, No.825 Zhangheng Road, Pudong New District, Shanghai 201203, China; [email protected] (Q.L.); [email protected] (J.W.); [email protected] (Q.H.); [email protected] (P.L.) 3 CAS Key Laboratory of Magnetic Resonance in Biological Systems, State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, National Centre for Magnetic Resonance in Wuhan, Wuhan Institute of Physics and Mathematics, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences, West No.30, Xiaohongshan, Wuchang District, Wuhan 430071, China * Correspondence: [email protected] (Y.L.); [email protected] (H.T.); [email protected] (H.X.) † These authors have contributed equally to this work. Citation: Li, G.; Lu, Q.; Wang, J.; Hu, Q.; Liu, P.; Yang, Y.; Li, Y.; Tang, H.; Abstract: Amomum tsaoko is a perennial herb belonging to Zingiberaceae. Its dried ripe fruit is Xie, H. Correlation Analysis of an important food additive, spice and materia medicai in Southeast Asia. For hundreds of years Compounds in Essential Oil of of cultivation, morphological variations have existed. -

Impatiens Oblongipetala (Balsaminaceae), a New Species from Yunnan, China
Impatiens oblongipetala (Balsaminaceae), a New Species from Yunnan, China Cong Yi-Yan and Liu Ke-Ming* Department of Botany, College of Life Science, Hunan Normal University, Changsha, 410081, Hunan, People’s Republic of China. *Author for correspondence: [email protected] ABSTRACT . A new species of Impatiens L., I. species (Liu Ke-Ming & Cong Yi-Yan 791376) were oblongipetala K. M. Liu & Y. Y. Cong (Balsamina- observed. Dried pollen grains and seeds from the ceae), from Yunnan Province, China, is described and holotype specimen were mounted on stubs with illustrated, including its seed and pollen micromor- double-sided adhesive tape and sputter-coated with phologies. This species is similar to I. lecomtei Hook. a layer of gold using the JFC-1600 Auto Fine Coater f. and I. weihsiensis Y. L. Chen, but differs by the (JEOL, Ltd., Tokyo, Japan). These coated materials white to slightly pink lateral sepals, the white lower were then examined and photographed using the JSM- sepal without purple striae, and the lateral united 6360LV scanning electron microscope (JEOL, Ltd.). petals with apically retuse distal lobes. Polar lengths and equatorial diameters were then Key words: Balsaminaceae, China, Impatiens, measured from 25 pollen grains and 25 seeds that IUCN Red List, Yunnan. were randomly chosen. Micromorphological charac- ters were described for pollen (Walker & Doyle, 1975; Impatiens L. is the larger of two genera in the Wang & Wang, 1983) and seeds (Liu et al., 2004), Balsaminaceae, with more than 900 species worldwide respectively. (Chen et al., 2008), and it occurs in tropical and subtropical mountains. Most of the species have very restricted distributions (Fischer & Rahelivololona, Impatiens oblongipetala K. -

Analysis of Earth Temperature Field and Geothermal Hazard Evaluation at Gaoligong Mountain Tunnel
Journal of Engineering Geology Volume XXXIX, No. 2, A bi-annual journal of ISEG December 2014 Analysis of earth temperature field and geothermal hazard evaluation at Gaoligong mountain tunnel Jihong Qi Mo Xu State Key Laboratory of Geohazard Prevention and Geoenvironment Protection, Chengdu University of Technology SKLGP,CDUT, Chengdu, China Abstract This paper analysis the geothermal hazard that may encounter during the construction of Gaoligong mountain tunnel located in the Southern part of Gaoligong Mountains. 1. Introduction: Gaoligong Mountain tunnel goes through the south part of Gaoligong Mountain. The research part of the tunnel is about 13.5 kilometers long (C12K197+337~C12K+868). The tunnel begins from the Huitong Bridge locating in Nujiang River, and ends near to the Long ling village. The maximum burying depth of this tunnel is about 1400 meters. Many warm springs appear in this area, some locate the north part of the tunnel, and some locate the south area of the tunnel. The temperature of warm springs can reaches to 100 ℃. This deeply buried tunnel may meet the warm groundwater or highly warm rock. The geothermal hazard can influent the construction safety, so much as make the tunnel route design impossible. For selecting reasonable and safety route design, researchers must predict the high geothermal distribution and analyze geothermal hazard which tunnel construction may meet. 2. Geological environment: Gaoligong Mountain locates in collision belt between Eurasian plate and Indian Ocean plate. The main tectonic extending direction is south to north and east to west. The research area includes Baoshan Mountain fold and Tengcong fold. These two folds are multiple ones.