Assessment of Neonatal Abstinence Syndrome: Standard Scoring of Infants Using the Finnegan Scoring Tool
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHALLENGING CASES: BIOPSYCHOSOCIAL PEDIATRICS Failure to Thrive in a 4-Month-Old Nursing Infant
CHALLENGING CASES: BIOPSYCHOSOCIAL PEDIATRICS Failure to Thrive in a 4-Month-Old Nursing Infant* CASE visits she was observed to respond quickly to visual Christine, a 4-month-old infant, is brought to the and auditory cues. There was no family history of office for a health-supervision visit by her mother, serious diseases. a 30-year-old emergency department nurse. The In that a comprehensive history and physical ex- mother, who is well known to the pediatrician to be amination did not indicate an organic cause, the a competent and attentive caregiver, appears unchar- pediatrician reasoned that the most likely cause of acteristically tired. They are accompanied by the 18- her failure to thrive was an unintentional caloric month-old and 4-year-old siblings because child care deprivation secondary to maternal stress and ex- was unavailable, and her husband has been out of haustion. Christine’s mother and the pediatrician town on business for the last 5 weeks. When the discussed how maternal stress, fatigue, and depres- pediatrician comments, “You look exhausted. It must sion could hinder the let-down reflex and reduce the be really tough to care for 3 young children,” the availability of an adequate milk supply, as well as mother appears relieved that attention was given to make it difficult to maintain regular feeding sessions. her needs. She reports that she has not been getting The pediatrician recommended obtaining help with much sleep because Christine wakes up for 2 or more child care and household responsibilities. The evening feedings and the toddler is now awakening mother was also encouraged to consume adequate at night. -

A Dose-Response Relationship Between Organic Mercury Exposure from Thimerosal-Containing Vaccines and Neurodevelopmental Disorders
Int. J. Environ. Res. Public Health 2014, 11, 9156-9170; doi:10.3390/ijerph110909156 OPEN ACCESS International Journal of Environmental Research and Public Health ISSN 1660-4601 www.mdpi.com/journal/ijerph Article A Dose-Response Relationship between Organic Mercury Exposure from Thimerosal-Containing Vaccines and Neurodevelopmental Disorders David A. Geier 1, Brian S. Hooker 2, Janet K. Kern 1,3, Paul G. King 4, Lisa K. Sykes 4 and Mark R. Geier 1,* 1 Institute of Chronic Illnesses, Inc., 14 Redgate Ct., Silver Spring, MD 20905, USA; E-Mails: [email protected] (D.A.G.); [email protected] (J.K.K.) 2 Department of Biology, Simpson University, 2211 College View Dr., Redding, CA 96003, USA; E-Mail: [email protected] 3 Department of Psychiatry, University of Texas Southwestern Medical Center at Dallas, 5353 Harry Hine Blvd., Dallas, TX 75390, USA 4 CoMeD, Inc., 14 Redgate Ct., Silver Spring, MD 20905, USA; E-Mails: [email protected] (P.G.K.); [email protected] (L.K.S.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-301-989-0548; Fax: +1-301-989-1543. Received: 12 July 2014; in revised form: 7 August 2014 / Accepted: 26 August 2014 / Published: 5 September 2014 Abstract: A hypothesis testing case-control study evaluated concerns about the toxic effects of organic-mercury (Hg) exposure from thimerosal-containing (49.55% Hg by weight) vaccines on the risk of neurodevelopmental disorders (NDs). Automated medical records were examined to identify cases and controls enrolled from their date-of-birth (1991–2000) in the Vaccine Safety Datalink (VSD) project. -

Neonatal Abstinence Syndrome Guideline
Neonatal Abstinence Syndrome MENU Introduction Incidence and risk factors 1. Risk factors for illicit drug use 2. Risk factors for adverse pregnancy outcomes 3. Risk factors for neonatal abstinence syndrome Consequences 1. Tobacco 2. Alcohol 3. Amphetamines 4. Cocaine and derivatives 5. Marijuana 6. Opiates 7. Polydrug use Diagnosis 1. Drug use in pregnancy 2. Testing of newborns 3. Diagnosis of withdrawal Interventions 1. Antenatal care a. Treatment of pregnant users of illicit drugs b. Smoking in pregnancy c. Support from caregivers for pregnant women abusing drugs 2. Postnatal care 3. Pharmacological treatment a. Opioid withdrawal: MORPHINE REGIMEN b. Non-opioid CNS depressants: PHENOBARBITONE REGIMEN c. Management of the vomiting baby Discharge The Drug use in pregnancy team Infant protection issues Follow-Up Keypoints References Introduction: Pregnant women using drugs have the same anxieties and expectations as other pregnant women. All women using drugs are entitled to accurate information, and to be treated sensitively in a non-judgmental manner. Maternal illicit drug use is a risk factor for adverse pregnancy and neonatal outcomes including preterm birth. Infants born to mothers using illicit drugs (and alcohol and tobacco) are at risk of the toxic effects of the drugs both in-utero and ex-utero; adverse pregnancy and neonatal outcomes related to the poor socioeconomic situations and lifestyle factors associated with illicit drug use; neonatal drug withdrawal; and the subsequent poor outcomes of infants reared in a socioeconomically disadvantaged environment. The Report of the US Preventative Services Task Force 1996 1 and the monograph review by Bell and Lau 2 1995 are used as background for the content of this guideline. -

Simplification of the Finnegan Neonatal Abstinence Scoring System: Retrospective Study of Two Institutions in the USA
Open Access Research BMJ Open: first published as 10.1136/bmjopen-2017-016176 on 27 September 2017. Downloaded from Simplification of the Finnegan Neonatal Abstinence Scoring System: retrospective study of two institutions in the USA Enrique Gomez Pomar,1 Loretta P Finnegan,2 Lori Devlin,3 Henrietta Bada,1 Vanessa A Concina,1 Katrina T Ibonia,1 Philip M Westgate4 To cite: Gomez Pomar E, ABSTRACT Strengths and limitations of this study Finnegan LP, Devlin L, et al. Objective To develop a simplified Finnegan Neonatal Simplification of the Finnegan Abstinence Scoring System (sFNAS) that will highly ► A simplified scoring system (simplified Finnegan Neonatal Abstinence Scoring correlate with scores ≥8 and ≥12 in infants being System: retrospective Neonatal Abstinence Scoring System (sFNAS)) is assessed with the FNAS. study of two institutions proposed developed from a large data set and cross- Design, setting and participants This is a in the USA. BMJ Open validated using a database from another institution. retrospective analysis involving 367 patients admitted to 2017;7:e016176. doi:10.1136/ ► Cutoff values are provided for the sFNAS which two level IV neonatal intensive care units with a total of bmjopen-2017-016176 predict the cutoff value. 40 294 observations. Inclusion criteria included neonates ► The retrospective nature of our study requires ► Prepublication history for with gestational age ≥37 0/7 weeks, who are being this paper is available online. prospective validation of the inter-rater reliability of assessed for neonatal abstinence syndrome (NAS) using To view these files, please visit the sFNAS and also the clinical validity of this tool. -

Intervention Ideas for Infants, Toddlers, Children, and Youth Impacted by Opioids
TOPICAL ISSUE BRIEF Intervention IDEAs for Infants, Toddlers, Children, and Youth Impacted by Opioids Overview Prevalence The abuse of opioids—such as heroin and various prescription drugs commonly prescribed for pain (e.g., oxycodone, hydrocodone, and fentanyl)—has rapidly gained attention across the United States as a public health crisis. Youth may be exposed to opioids in a variety of ways, including but not limited to infants born to mothers who took opioids during pregnancy; children mistakenly consuming opioids (perhaps thinking they are candy); teenagers taking opioids from an illicit street supply; or, more commonly, teenagers being given opiates for free by a friend or relative.1,2 Even appropriate opioid use among adolescents and young adults to treat pain may slightly increase their risk of later opioid misuse.3 The Centers for Disease Control and Prevention (CDC) have identified opioid abuse as an epidemic, noting that opioids impact and affect all communities and age groups.4 Between 1997 and 2012, 13,052 children were hospitalized for poisonings caused by oxycodone, Percocet (a combination of acetaminophen and oxycodone), codeine, and other prescription opioids.5 Every 25 minutes, an infant is born suffering from opioid withdrawal, and an estimated 21,732 infants were born in 2012 with neonatal abstinence syndrome (NAS), a drug withdrawal syndrome.6 According to data from 2014, approximately 28,000 adolescents had used heroin within the past year, 16,000 were current heroin users, and 18,000 had a heroin use disorder.7 At the same time, relatively few data are available about the effects of opiates on the U.S. -

Child and Adolescent Needs and Strengths: Early Childhood
Child and Adolescent Needs and Strengths: Early Childhood CANS: EC Supplemental Guide A Support for the CANS (Early Childhood) Information Integration Tool for Young Children Ages Birth to Five SET is part of the System of Care Initiative, within the Allegheny County Department of Human Services, Office of Behavioral Health Stacey Cornett, Copyright 2007. Child and Adolescent Needs and Strengths: Early Childhood (CANS:EC) Section One: Child Strengths This section focuses on the attributes, traits, talents and skills of the child that can be useful in developing a strength-based treatment plan. Within a systems of care approach identifying strengths is considered an essential part of the process (Miles, P., Burns, E.J., Osher, T.W., Walker, J.S., 2006). A focus on strengths that both the child and the family have to offer allows for a climate of hope and optimism and has been proven to engage families at a higher level. Strength-based assessment has been defined as, “the measurement of those emotional and behavioral skills, competencies, and characteristics that create a sense of personal accomplishment; contribute to satisfying relationships with family members, peers, and adults; enhance one’s ability to deal with adversity and stress; and promote one’s personal, social, and academic development” (Epstein & Sharma, 1998, p.3). The benefits of a strength- based assessment include involving parents and children in a service planning experience that builds on what a child and family are doing well, facilitates positive expectations for the child, and empowers family members to take responsibility for the decisions that will affect their child (Johnson & Friedman, 1991; Saleebey, 1992). -

The Child with Failure to Thrive
The Child with Failure to Thrive Bonnie Proulx, MSN, APRN, PNP Pediatric Gastroenterology Children’s Hospital at Dartmouth Manchester, NH DISCLOSURES None of the planners or presenters of this session have disclosed any conflict or commercial interest The Child with Failure to Thrive OBJECTIVES: 1. Review assessment and appropriate diagnostic testing for children who fail meet expected growth 2. Discuss various causes of failure to thrive. 3. Identify appropriate referrals for children with failure to thrive. Definition • Inadequate physical growth diagnosed by observation of growth over time on a standard growth chart Definition • More than 2 standard deviations below mean for age • Downward trend in growth crossing 2 major percentiles in a short time • Comparison of height and weight • Not a diagnosis but a finding Pitfalls • Some children are just tiny • Familial short stature children may cross percentiles to follow genetic predisposition • Normal small children may be diagnosed later given already tiny Frequency • Affects 5-10% of children under 5 in developing countries • Peak incidence between 9 and 24 months of age • Uncommon after the age of 5 What is normal growth • Birth to 6 months, a baby may grow 1/2 to 1 inch (about 1.5 to 2.5 centimeters) a month • 6 to 12 months, a baby may grow 3/8 inch (about 1 centimeter) a month • 2nd year of life 4-6 inches • 3rd year 3-4 inches • Annual growth until pre-puberty 2-3 inches per year Normal weight gain • Birth to 6 months-gain 5 to 7 ounces a week. • 6 to 12 months -gain 3 to 5 ounces (about 85 to 140 grams) a week. -

Practice Resource: CARE of the NEWBORN EXPOSED to SUBSTANCES DURING PREGNANCY
Care of the Newborn Exposed to Substances During Pregnancy Practice Resource for Health Care Providers November 2020 Practice Resource: CARE OF THE NEWBORN EXPOSED TO SUBSTANCES DURING PREGNANCY © 2020 Perinatal Services BC Suggested Citation: Perinatal Services BC. (November 2020). Care of the Newborn Exposed to Substances During Pregnancy: Instructional Manual. Vancouver, BC. All rights reserved. No part of this publication may be reproduced for commercial purposes without prior written permission from Perinatal Services BC. Requests for permission should be directed to: Perinatal Services BC Suite 260 1770 West 7th Avenue Vancouver, BC V6J 4Y6 T: 604-877-2121 F: 604-872-1987 [email protected] www.perinatalservicesbc.ca This manual was designed in partnership by UBC Faculty of Medicine’s Division of Continuing Professional Development (UBC CPD), Perinatal Services BC (PSBC), BC Women’s Hospital & Health Centre (BCW) and Fraser Health. Content in this manual was derived from module 3: Care of the newborn exposed to substances during pregnancy in the online module series, Perinatal Substance Use, available from https://ubccpd.ca/course/perinatal-substance-use Perinatal Services BC Care of the Newborn Exposed to Substances During Pregnancy ii Limitations of Scope Iatrogenic opioid withdrawal: Infants recovering from serious illness who received opioids and sedatives in the hospital may experience symptoms of withdrawal once the drug is discontinued or tapered too quickly. While these infants may benefit from the management strategies discussed in this module, the ESC Care Tool is intended for newborns with prenatal substance exposure. Language A note about gender and sexual orientation terminology: In this module, the terms pregnant women and pregnant individual are used. -
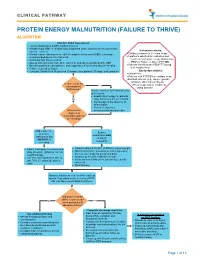
Protein Energy Malnutrition (Failure to Thrive) Algorithm
CLINICAL PATHWAY PROTEIN ENERGY MALNUTRITION (FAILURE TO THRIVE) ALGORITHM Conduct Initial Assessment • History and physical (H&P), nutrition focused • Weight height, BMI, % of ideal body weight and exam: assess severity (symmetric edema = severe) Inclusion criteria: • • Consider basic labs based on H&P; A complete blood count (CBC) is strongly Children newborn to 21 years of age • recommended due to risk of anemia Inpatients admitted for evaluation and • Additional labs based on H&P treatment of Protein Energy Malnutrition • Assess micronutrients: iron, zinc, vitamin D, and others as indicated by H&P (PEM) or Failure to thrive (FTT) OR • • Baseline potassium, phosphorus, and magnesium if concerned about re-feeding Patients identified with PEM/FTT during • Calorie count up to 3 days their hospital stay. • Consults: Social Work, Registered Dietician, Occupational Therapy, and Lactation Exclusion criteria: • Outpatients • Patients with FTT/PEM secondary to an identified concern (e.g., cancer, genetic condition, other chronic illness). Is there a risk for •Pts w/ suspected or confirmed micronutrient Yes eating disorder deficiencies? Initiate treatment for micronutrients deficiencies: • Empiric zinc therapy for patients No older than 6 months for 1 month • Iron therapy in the absence of inflammation • Vitamin D and other What are the micronutrients based on labs degrees of malnutrition and risk of refeeding? Mild, moderate, Severe or severe malnutrition AND malnutrition but at risk of NO RISK of refeeding refeeding • • Initiate feeding per recommended Initiate feeding at 30-50% of RDA for current weight • daily allowance (RDA) for current Monitor potassium, phosphorus, and magnesium weight and age once to twice a day for a total of 4 days • • Use PO route if patient is able to Advance by 10-20% if labs are normal • take 70% of estimated calories If labs abnormal hold off on advancing feed until orally corrected • Start thiamine Advance calories to meet level for catch up growth. -

Parenting Children Who Have Been Exposed to Methamphetamine
Non-Return Information Packet Assisting families on their lifelong journey Parenting Children Who Have Been Exposed to Methamphetamine A Brief Guide for Adoptive, Guardianship, and Foster Parents Oregon Post Adoption Resource Center 2950 SE Stark Street, Suite 130 Portland, Oregon 97214 503-241-0799 800-764-8367 503-241-0925 Fax [email protected] www.orparc.org ORPARC is a contracted service of the Oregon Department of Human Services. Please do not reproduce without permission. PARENTING CHILDREN WHO HAVE BEEN EXPOSED TO METHAMPHETAMINE A BRIEF GUIDE FOR ADOPTIVE, GUARDIANSHIP AND FOSTER PARENTS Table of Contents Introduction: ............................................................................................................. 1 Part I: Methamphetamine: An Overview ........................................................... 2 What is meth? What are its effects on the user? How prevalent is meth use? How is meth addiction treated? Part II: Meth’s Effects on Children ...................................................................... 7 What are the prenatal effects of exposure? What are the postnatal effects of prenatal exposure? What are the environmental effects on children? Part III: Parenting Meth-Exposed Children ....................................................... 11 Guiding principles Age-specific suggestions Part IV: Reprinted Articles .................................................................................. 20 Appendix A: Recommended Resources Appendix B: Sources Page i PARENTING CHILDREN WHO HAVE -

Prenatal Cocaine Exposure and Infant Cognition
Infant Behavior & Development 28 (2005) 431–444 Prenatal cocaine exposure and infant cognition Lynn T. Singer a,∗, Laurie J. Eisengart a, Sonia Minnes a, Julia Noland b, Arthur Jey a, Courtney Lane a, Meeyoung O. Min a a Department of Pediatrics, Case Western Reserve University, The Triangle Building, 11400 Euclid Avenue, Suite 250-A, Cleveland, OH 44106, USA b Vanderbilt University, Nashville, TN, USA Received 9 November 2004; received in revised form 8 March 2005; accepted 24 March 2005 Abstract The present study examined the relationship of prenatal cocaine exposure to infant information processing in the first year of life. In a prospective, longitudinal study of 177 cocaine-exposed and 175 non-exposed infants, the Fagan Test of Infant Intelligence (FTII) was used to measure attention, visual recognition memory and information processing speed at 6.5 and 12 months of age. Groups were compared over time using mixed linear model analyses. Prenatal cocaine exposure predicted poorer visual recognition memory at 12 months, with exposed infants ob- taining lower mean scores and a higher percentage of scores in the risk range. Across exposure groups, information processing speed increased with age, demonstrating a developmental effect. Tobacco and marijuana exposures were related to faster looking times, which did not relate to visual recognition memory. Cognitive deficits and attentional problems noted in prior studies of cocaine-exposed children at later ages may be detectable in infancy. © 2005 Elsevier Inc. All rights reserved. Keywords: Cocaine; Infants; Visual recognition; Memory; Fagan Test of Infant Intelligence 1. Introduction During the 1980s, cocaine use reached epidemic levels in the United States among pregnant women in urban areas, subsequently exposing hundreds of thousands of children to cocaine prenatally (National ∗ Corresponding author. -

Paediatrics at a Glance
Paediatrics at a Glance Lawrence Miall Mary Rudolf Malcolm Levene Blackwell Science Paediatrics at a Glance This book is dedicated to our children Charlie, Mollie, Rosie Aaron, Rebecca Alysa, Katie, Ilana, Hannah, David and all those children who enlightened and enlivened us during our working lives. Paediatrics at a Glance LAWRENCE MIALL MB BS, BSc, MMedSc, MRCP, FRCPCH Consultant Neonatologist and Honorary Senior Lecturer Neonatal Intensive Care Unit St James’s University Hospital Leeds MARY RUDOLF MB BS BSc DCH FRCPCH FAAP Consultant Paeditrician in Community Child Health Leeds Community Children’s Services Belmont House Leeds MALCOLM LEVENE MD FRCP FRCPCH FMedSc Professor of Paediatrics School of Medicine Leeds General Infirmary Leeds Blackwell Science © 2003 by Blackwell Science Ltd a Blackwell Publishing company Blackwell Science, Inc., 350 Main Street, Malden, Massachusetts 02148-5018, USA Blackwell Science Ltd, Osney Mead, Oxford OX2 0EL, UK Blackwell Science Asia Pty Ltd, 550 Swanston Street, Carlton, Victoria 3053, Australia Blackwell Wissenschafts Verlag, Kurfürstendamm 57, 10707 Berlin, Germany The right of the Authors to be identified as the Authors of this Work has been asserted in accordance with the Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. First published 2003 Library of Congress Cataloging-in-Publication Data Miall, Lawrence. Paediatrics at a glance/Lawrence Miall, Mary Rudolf, Malcolm Levene.