ADAM15 Supports Prostate Cancer Metastasis by Modulating Tumor Cell–Endothelial Cell Interaction Abdo J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Negatively Regulated by ADAM15 TRIF-Mediated TLR3 and TLR4
TRIF-Mediated TLR3 and TLR4 Signaling Is Negatively Regulated by ADAM15 Suaad Ahmed, Ashwini Maratha, Aisha Qasim Butt, Enda Shevlin and Sinead M. Miggin This information is current as of September 27, 2021. J Immunol 2013; 190:2217-2228; Prepublished online 30 January 2013; doi: 10.4049/jimmunol.1201630 http://www.jimmunol.org/content/190/5/2217 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2013/01/30/jimmunol.120163 Material 0.DC1 References This article cites 57 articles, 20 of which you can access for free at: http://www.jimmunol.org/ http://www.jimmunol.org/content/190/5/2217.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 27, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2013 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology TRIF-Mediated TLR3 and TLR4 Signaling Is Negatively Regulated by ADAM15 Suaad Ahmed, Ashwini Maratha, Aisha Qasim Butt, Enda Shevlin, and Sinead M. -

Conservation and Divergence of ADAM Family Proteins in the Xenopus Genome
Wei et al. BMC Evolutionary Biology 2010, 10:211 http://www.biomedcentral.com/1471-2148/10/211 RESEARCH ARTICLE Open Access ConservationResearch article and divergence of ADAM family proteins in the Xenopus genome Shuo Wei*1, Charles A Whittaker2, Guofeng Xu1, Lance C Bridges1,3, Anoop Shah1, Judith M White1 and Douglas W DeSimone1 Abstract Background: Members of the disintegrin metalloproteinase (ADAM) family play important roles in cellular and developmental processes through their functions as proteases and/or binding partners for other proteins. The amphibian Xenopus has long been used as a model for early vertebrate development, but genome-wide analyses for large gene families were not possible until the recent completion of the X. tropicalis genome sequence and the availability of large scale expression sequence tag (EST) databases. In this study we carried out a systematic analysis of the X. tropicalis genome and uncovered several interesting features of ADAM genes in this species. Results: Based on the X. tropicalis genome sequence and EST databases, we identified Xenopus orthologues of mammalian ADAMs and obtained full-length cDNA clones for these genes. The deduced protein sequences, synteny and exon-intron boundaries are conserved between most human and X. tropicalis orthologues. The alternative splicing patterns of certain Xenopus ADAM genes, such as adams 22 and 28, are similar to those of their mammalian orthologues. However, we were unable to identify an orthologue for ADAM7 or 8. The Xenopus orthologue of ADAM15, an active metalloproteinase in mammals, does not contain the conserved zinc-binding motif and is hence considered proteolytically inactive. We also found evidence for gain of ADAM genes in Xenopus as compared to other species. -

ADAM15 Targets MMP9 Activity to Promote Lung Cancer Cell Invasion
ONCOLOGY REPORTS 34: 2451-2460, 2015 ADAM15 targets MMP9 activity to promote lung cancer cell invasion DAN-DAN DONG1, HUI ZHOU2 and GAO LI3 1Department of Pathology, Sichuan Academy of Medical Sciences, Sichuan Provincial People's Hospital, Chengdu, Sichuan 610072; 2Department of Thoracic Medicine, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, Hunan 410013; 3Department of Thoracic Surgery, Hainan General Hospital, Haikou, Hainan 570311, P.R. China Received June 17, 2015; Accepted July 20, 2015 DOI: 10.3892/or.2015.4203 Abstract. ADAM15 is a membrane-associated proteinase bound cell surface glycoproteins (1). These zinc-dependent belonging to a disintegrin and metalloproteinase (ADAM) proteases contain an amino terminal metalloproteinase and a family. Recent studies suggested that ADAM15 is overex- disintegrin domain, a cysteine-rich and an EGF-like sequence, pressed in several types of cancer and is involved in metastatic a transmembrane and a cytoplasmic domain (2). It has been tumor progression. However, the function of ADAM15 in proven that 13 members of ADAM family are catalytically non-small cell lung cancer (NSCLC) is currently unknown. In active through their metalloproteinase domain. These catalytic the present study, we found that high expression of ADAM15 members play an important role in mediating extracellular was associated with decreased overall survival (OS) and matrix protein degradation and shedding of growth factors, disease-free survival (DFS) in NSCLC patients. Furthermore, cytokines and adhesion molecules. Besides, the disintegrin shRNA-mediated knockdown of ADAM15 attenuated cell domain of ADAMs is also involved in binding to integrins migration and invasion. Mechanistic study demonstrated that to mediate cell-cell and cell-matrix interactions (3). -

A Disintegrin and Metalloprotease 15 Is Expressed on Rheumatoid Arthritis Synovial Tissue Endothelial Cells and May Mediate Angiogenesis
cells Article A Disintegrin and Metalloprotease 15 is Expressed on Rheumatoid Arthritis Synovial Tissue Endothelial Cells and may Mediate Angiogenesis Shinichiro Nishimi, Takeo Isozaki * , Kuninobu Wakabayashi, Hiroko Takeuchi and Tsuyoshi Kasama Division of Rheumatology, Department of Medicine, Showa University School of Medicine, Tokyo 142-8555, Japan; [email protected] (S.N.); [email protected] (K.W.); [email protected] (H.T.); [email protected] (T.K.) * Correspondence: [email protected]; Tel.: +813(3784)-8942; Fax: +813(3784)-8946 Received: 7 November 2018; Accepted: 5 January 2019; Published: 9 January 2019 Abstract: A disintegrin and metalloprotease 15 (ADAM15) is involved in several malignancies. In this study, we investigated the role of ADAM15 in rheumatoid arthritis (RA) angiogenesis. Soluble ADAM15 (s-ADAM15) in serum from RA and normal (NL) subjects was measured using ELISA. To determine membrane-anchored ADAM15 (ADAM15) expression in RA synovial tissues, immunohistochemistry was performed. To examine the role of ADAM15 in angiogenesis, we performed in vitro Matrigel assays and monocyte adhesion assays using human umbilical vein endothelial cells (HUVECs) transfected with ADAM15 siRNA. Finally, to investigate whether angiogenic mediators were affected by ADAM15, cytokines in ADAM15 siRNA-transfected HUVEC-conditioned medium were measured. ADAM15 was significantly higher in RA serum than in NL serum. ADAM15 was also expressed on RAST endothelial cells. ADAM15 siRNA-treated HUVECs had decreased EC tube formation in response to RA synovial fluids compared with non-treated HUVECs. The adhesion index of ADAM15 siRNA-transfected HUVECs was significantly lower than the adhesion index of control siRNA-transfected HUVECs. -

Novel Alternatively Spliced ADAM8 Isoforms Contribute to the Aggressive Bone Metastatic Phenotype of Lung Cancer
Oncogene (2010) 29, 3758–3769 & 2010 Macmillan Publishers Limited All rights reserved 0950-9232/10 www.nature.com/onc ORIGINAL ARTICLE Novel alternatively spliced ADAM8 isoforms contribute to the aggressive bone metastatic phenotype of lung cancer I Herna´ndez1, JL Moreno2, C Zandueta1, L Montuenga3,4 and F Lecanda1 1Adhesion and Metastasis Laboratory, Division of Oncology, University of Navarra, Pamplona, Spain; 2Department of Orthopaedics, University of Maryland School of Medicine, Baltimore, MD, USA; 3Department of Histology and Pathology, School of Medicine, University of Navarra, Pamplona, Spain and 4Biomarkers Laboratory, Center for Applied Biomedical Research (CIMA), University of Navarra, Pamplona, Spain ADAMs (a disintegrin and metalloprotease) are trans- survival rates for lung cancer are o15% in all developed membrane proteins involved in a variety of physiological countries. It is estimated that 30–40% of lung cancer processes and tumorigenesis. Recently, ADAM8 has been patients with advanced NSCLC suffer from bone associated with poor prognosis of lung cancer. However, metastasis (Coleman, 1997). Patients with bone metas- its contribution to tumorigenesis in the context of lung tasis experience pain, metabolic syndromes and spinal cancer metastasis remains unknown. Native ADAM8 cord compression associated with pathological fractures expression levels were lower in lung cancer cell lines. as a consequence of osteolytic lesions. In contrast, we identified and characterized two novel ADAMs (a disintegrin and metalloprotease) form a spliced isoforms encoding truncated proteins, D18a and large family of cell-surface proteins, which are char- D140, which were present in several tumor cell lines and not acterized by disintegrin and metalloproteinase domains, in normal cells. Overexpression of D18a protein resulted in that possess adhesive properties and proteolytic activ- enhanced invasive activity in vitro.ADAM8anditsD140 ities, respectively (Lu et al., 2007). -
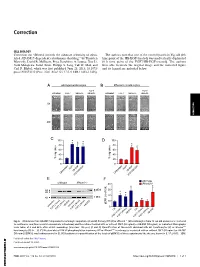
Irhom2 Controls the Substrate Selectivity of Stimulated ADAM17-Dependent Ectodomain Shedding
Correction CELL BIOLOGY Correction for “iRhom2 controls the substrate selectivity of stimu- The authors note that one of the control panels in Fig. 6B (0 h lated ADAM17-dependent ectodomain shedding,” by Thorsten time point of the HB-EGF-treated) was inadvertently duplicated Maretzky, David R. McIlwain, Priya Darshinee A. Issuree, Xue Li, (0 h time point of the FGF7/HB-EGF-treated). The authors Jordi Malapeira, Sadaf Amin, Philipp A. Lang, Tak W. Mak, and were able to locate the original image and the corrected figure Carl P. Blobel, which was first published June 25, 2013; 10.1073/ and its legend are included below. pnas.1302553110 (Proc. Natl. Acad. Sci. U.S.A. 110,11433–11438). AB CORRECTION C D E F Fig. 6. iRhom2 controls ADAM17-dependent keratinocyte migration. (A and B) Primary WT (A)oriRhom2−/− (B) keratinocytes from 12-wk-old animals were cultured to confluence, and then a scratch wound was introduced, and the cultures treated with or without FGF7 (50 ng/mL) or HB-EGF (50 ng/mL), as indicated. Micrographs − − were taken at 0 and 48 h after scratch wounding. (Scale bar: 100 μm.) (C and D) Quantification of the results obtained with WT keratinocytes (C)oriRhom2 / − − keratinocytes (D)(n = 3). (E) Western blot of ERK1/2 phosphorylation in primary WT or iRhom2 / keratinocytes incubated with or without FGF7 (20 ng/mL) or HB-EGF (50 ng/mL) (ERK1/2 was loading control in E). (F) Densitometric quantification of the levels of pERK1/2 of three experiments like the one shown in E.*P ≤ 0.05; ±SEM. -

ADAM15/PTK6/Cmet Interplay: Promotors of Prostate Cancer
ADAM15/PTK6/cMET interplay: Promotors of prostate cancer progression Melanie Hurtz, MSc. September 2017 School of Medicine Cardiff University Cardiff, CF14 4XN Cardiff, United Kingdom A thesis submitted in partial fulfilment of the requirements of the degree of Doctor of Philosophy (PhD) DECLARATION This Work has not been submitted in substance for any other degree or award at this or any other university or place of learning, nor is being submitted concurrently in candidature for any degree or other aWard. Signed ………………………………… (candidate) Date ………………………… I STATEMENT 1 This thesis is being submitted in partial fulfilment of the requirements for the degree of PhD Signed ………………………………………(candidate) Date ………………………… STATEMENT 2 This thesis is the result of my oWn independent Work/investigation, except Where otherWise stated. Other sources are acknowledged by explicit references. The views expressed are my own. Signed ………………………………………(candidate) Date ………………………… STATEMENT 3 I hereby give consent for my thesis, if accepted, to be available for photocopying and for inter-library loan, and for the title and summary to be made available to outside organisations. Signed ………………………………………(candidate) Date ………………………… STATEMENT 4: PREVIOUSLY APPROVED BAR ON ACCESS I hereby give consent for my thesis, if accepted, to be available for photocopying and for inter-library loans after expiry of a bar on access previously approved by the Academic Standards & Quality Committee. Signed ………………………………………(candidate) Date ……………………… II Σε αυτό που βρήκα εδώ στο καρντιφ, και το μικρό μπόνους Für Joko, und all die guten Tage zum Fliegen Für Mama und Papa III Acknowledgments This thesis Would have been impossible Without the support of my supervisor’s Dr Zara Poghosyan and Dr Vera Knäuper. -

The Adams Family of Metalloproteases: Multidomain Proteins with Multiple Functions
Downloaded from genesdev.cshlp.org on September 26, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW The ADAMs family of metalloproteases: multidomain proteins with multiple functions Darren F. Seals and Sara A. Courtneidge1 Van Andel Research Institute, Grand Rapids, Michigan 49503, USA The ADAMs family of transmembrane proteins belongs diseases such as arthritis and cancer (Chang and Werb to the zinc protease superfamily. Members of the family 2001). Adamalysins are similar to the matrixins in their have a modular design, characterized by the presence of metalloprotease domains, but contain a unique integrin metalloprotease and integrin receptor-binding activities, receptor-binding disintegrin domain (Fig. 1). It is the and a cytoplasmic domain that in many family members presence of these two domains that give the ADAMs specifies binding sites for various signal transducing pro- their name (a disintegrin and metalloprotease). The do- teins. The ADAMs family has been implicated in the main structure of the ADAMs consists of a prodomain, a control of membrane fusion, cytokine and growth factor metalloprotease domain, a disintegrin domain, a cyste- shedding, and cell migration, as well as processes such as ine-rich domain, an EGF-like domain, a transmembrane muscle development, fertilization, and cell fate determi- domain, and a cytoplasmic tail. The adamalysins sub- nation. Pathologies such as inflammation and cancer family also contains the class III snake venom metallo- also involve ADAMs family members. Excellent reviews proteases and the ADAM-TS family, which although covering various facets of the ADAMs literature-base similar to the ADAMs, can be distinguished structurally have been published over the years and we recommend (Fig. -

ADAM8 As a Drug Target in Pancreatic Cancer
ARTICLE Received 19 Feb 2014 | Accepted 24 Dec 2014 | Published 28 Jan 2015 DOI: 10.1038/ncomms7175 ADAM8 as a drug target in pancreatic cancer Uwe Schlomann1,2, Garrit Koller1, Catharina Conrad2, Taheera Ferdous1, Panagiota Golfi1, Adolfo Molejon Garcia1, Sabrina Ho¨fling1, Maddy Parsons3, Patricia Costa4, Robin Soper4, Maud Bossard4, Thorsten Hagemann4, Rozita Roshani4, Norbert Sewald5, Randal R. Ketchem6, Marcia L. Moss7, Fred H. Rasmussen7, Miles A. Miller8, Douglas A. Lauffenburger8, David A. Tuveson9, Christopher Nimsky2 &Jo¨rg W. Bartsch1,2 Pancreatic ductal adenocarcinoma (PDAC) has a grim prognosis with o5% survivors after 5 years. High expression levels of ADAM8, a metalloprotease disintegrin, are correlated with poor clinical outcome. We show that ADAM8 expression is associated with increased migration and invasiveness of PDAC cells caused by activation of ERK1/2 and higher MMP activities. For biological function, ADAM8 requires multimerization and associates with b1 integrin on the cell surface. A peptidomimetic ADAM8 inhibitor, BK-1361, designed by structural modelling of the disintegrin domain, prevents ADAM8 multimerization. In PDAC cells, BK-1361 affects ADAM8 function leading to reduced invasiveness, and less ERK1/2 and MMP activation. BK-1361 application in mice decreased tumour burden and metastasis of implanted pancreatic tumour cells and provides improved metrics of clinical symptoms and survival in a KrasG12D-driven mouse model of PDAC. Thus, our data integrate ADAM8 in pancreatic cancer signalling and validate ADAM8 as a target for PDAC therapy. 1 King’s College London, Institute for Pharmaceutical Science and KCLDI, London SE1 9RT, UK. 2 Department of Neurosurgery, Marburg University, , Baldingerstrasse, 35033 Marburg, Germany. -

Autocrine IFN Signaling Inducing Profibrotic Fibroblast Responses By
Downloaded from http://www.jimmunol.org/ by guest on September 23, 2021 Inducing is online at: average * The Journal of Immunology , 11 of which you can access for free at: 2013; 191:2956-2966; Prepublished online 16 from submission to initial decision 4 weeks from acceptance to publication August 2013; doi: 10.4049/jimmunol.1300376 http://www.jimmunol.org/content/191/6/2956 A Synthetic TLR3 Ligand Mitigates Profibrotic Fibroblast Responses by Autocrine IFN Signaling Feng Fang, Kohtaro Ooka, Xiaoyong Sun, Ruchi Shah, Swati Bhattacharyya, Jun Wei and John Varga J Immunol cites 49 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2013/08/20/jimmunol.130037 6.DC1 This article http://www.jimmunol.org/content/191/6/2956.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2013 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 23, 2021. The Journal of Immunology A Synthetic TLR3 Ligand Mitigates Profibrotic Fibroblast Responses by Inducing Autocrine IFN Signaling Feng Fang,* Kohtaro Ooka,* Xiaoyong Sun,† Ruchi Shah,* Swati Bhattacharyya,* Jun Wei,* and John Varga* Activation of TLR3 by exogenous microbial ligands or endogenous injury-associated ligands leads to production of type I IFN. -

Quantitative Proteomics Reveals Changes Induced by TIMP-3 on Cell Membrane Composition and Novel Metalloprotease Substrates
International Journal of Molecular Sciences Article Quantitative Proteomics Reveals Changes Induced by TIMP-3 on Cell Membrane Composition and Novel Metalloprotease Substrates Anna Paola Carreca 1,† , Veronica Maria Pravatà 2,3,† , Danilo D’Apolito 4,5 , Simone Bonelli 1, Matteo Calligaris 1,6, Elisa Monaca 7 , Stephan A. Müller 3, Stefan F. Lichtenthaler 3,8,9 and Simone Dario Scilabra 1,* 1 Proteomics Group of Fondazione Ri.MED, Department of Research IRCCS ISMETT, via Ernesto Tricomi 5, 90145 Palermo, Italy; [email protected] (A.P.C.); [email protected] (S.B.); [email protected] (M.C.) 2 Division of Gene Regulation and Expression, School of Life Sciences, University of Dundee, Dundee DD1 5EH, UK; [email protected] 3 German Center for Neurodegenerative Diseases (DZNE), Feodor-Lynen Strasse 17, 81377 Munich, Germany; [email protected] (S.A.M.); [email protected] (S.F.L.) 4 Unità di Medicina di Laboratorio e Biotecnologie Avanzate, IRCCS-ISMETT (Istituto Mediterraneo per i Trapianti e Terapie ad Alta Specializzazione), Via E. Tricomi 5, 90127 Palermo, Italy; [email protected] 5 Unità Prodotti Cellulari (GMP), Fondazione Ri.MED c/o IRCCS-ISMETT, Via E. Tricomi 5, 90127 Palermo, Italy 6 Department of Pharmacy, University of Pisa, Via Bonanno 6, 56126 Pisa, Italy 7 Fondazione Ri.MED, 90133 Palermo, Italy; [email protected] Citation: Carreca, A.P.; Pravatà, V.M.; 8 Neuroproteomics, Klinikum rechts der Isar, Technische Universität München, 81675 Munich, Germany D’Apolito, D.; Bonelli, S.; Calligaris, 9 Munich Cluster for Systems Neurology (SyNergy), 81377 Munich, Germany M.; Monaca, E.; Müller, S.A.; * Correspondence: [email protected]; Tel.: +39-(0)91-219-2430 Lichtenthaler, S.F.; Scilabra, S.D. -

The Role of Proteases in Epithelial-To-Mesenchymal Cell Transitions in Cancer
Cancer and Metastasis Reviews (2019) 38:431–444 https://doi.org/10.1007/s10555-019-09808-2 The role of proteases in epithelial-to-mesenchymal cell transitions in cancer Julia Mitschke1 & Ulrike C. Burk1 & Thomas Reinheckel1,2,3 Published online: 3 September 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Changing the characteristics of cells from epithelial states to mesenchymal properties is a key process involved in developmental and physiological processes as well as in many diseases with cancer as the most prominent example. Nowadays, a great deal of work and literature concerns the understanding of the process of epithelial-to-mesenchymal transition (EMT) in terms of its molecular regulation and its implications for cancer. Similar statements can certainly be made regarding the investigation of the more than 500 proteases typically encoded by a mammalian genome. Specifically, the impact of proteases on tumor biology has been a long-standing topic of interest. However, although EMT actively regulates expression of many proteases and proteolytic enzymes are clearly involved in survival, division, differentiation, and movements of cells, information on the diverse roles of proteases in EMT has been rarely compiled. Here we aim to conceptually connect the scientific areas of “EMT” and “protease” research by describing how several important classes of proteolytic enzymes are regulated by EMT and how they are involved in initiation and execution of the EMT program. To do so, we briefly introduce the evolving key features of EMT and its regulation followed by discussion of protease involvement in this process. Keywords Metalloprotease . Deubiquitinating enzyme .