Testing for Amphetamine and Methamphetamine Abuse Using
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

House Bill No. 2191
SECOND REGULAR SESSION HOUSE BILL NO. 2191 99TH GENERAL ASSEMBLY INTRODUCED BY REPRESENTATIVE QUADE. 5582H.01I D. ADAM CRUMBLISS, Chief Clerk AN ACT To repeal section 579.060, RSMo, and to enact in lieu thereof one new section relating to controlled substances, with penalty provisions. Be it enacted by the General Assembly of the state of Missouri, as follows: Section A. Section 579.060, RSMo, is repealed and one new section enacted in lieu 2 thereof, to be known as section 579.060, to read as follows: 579.060. 1. A person commits the offense of unlawful sale, distribution, or purchase of 2 over-the-counter methamphetamine precursor drugs if he or she knowingly: 3 (1) Sells, distributes, dispenses, or otherwise provides any number of packages of any 4 drug product containing detectable amounts of ephedrine, levomethamphetamine, 5 phenylpropanolamine, propylhexedrine, or pseudoephedrine, or any of their salts, optical 6 isomers, or salts of optical isomers, in a total amount greater than nine grams to the same 7 individual within a thirty-day period, unless the amount is dispensed, sold, or distributed 8 pursuant to a valid prescription; or 9 (2) Purchases, receives, or otherwise acquires within a thirty-day period any number of 10 packages of any drug product containing any detectable amount of ephedrine, 11 levomethamphetamine, phenylpropanolamine, propylhexedrine, or pseudoephedrine, or any 12 of their salts or optical isomers, or salts of optical isomers in a total amount greater than nine 13 grams, without regard to the number of transactions, unless the amount is purchased, received, 14 or acquired pursuant to a valid prescription; or 15 (3) Purchases, receives, or otherwise acquires within a twenty-four-hour period any 16 number of packages of any drug product containing any detectable amount of ephedrine, 17 levomethamphetamine, phenylpropanolamine, propylhexedrine, or pseudoephedrine, or any EXPLANATION — Matter enclosed in bold-faced brackets [thus] in the above bill is not enacted and is intended to be omitted from the law. -

Therapeutic Potential of Nicotinamide Adenine Dinucleotide (NAD) T ∗ Marta Arenas-Jala,B, , J.M
European Journal of Pharmacology 879 (2020) 173158 Contents lists available at ScienceDirect European Journal of Pharmacology journal homepage: www.elsevier.com/locate/ejphar Therapeutic potential of nicotinamide adenine dinucleotide (NAD) T ∗ Marta Arenas-Jala,b, , J.M. Suñé-Negrea, Encarna García-Montoyaa a Pharmacy and Pharmaceutical Technology Department (Faculty of Pharmacy and Food Sciences), University of Barcelona, Barcelona, Spain b ICN2 – Catalan Institute of Nanoscience and Nanotechnology (Autonomous University of Barcelona), Bellaterra (Barcelona), Spain ARTICLE INFO ABSTRACT Keywords: Nicotinamide adenine nucleotide (NAD) is a small ubiquitous hydrophilic cofactor that participates in several NAD aspects of cellular metabolism. As a coenzyme it has an essential role in the regulation of energetic metabolism, Metabolism but it is also a cosubstrate for enzymes that regulate fundamental biological processes such as transcriptional Therapeutic potential regulation, signaling and DNA repairing among others. The fluctuation and oxidative state of NAD levels reg- Drug discovery ulate the activity of these enzymes, which is translated into marked effects on cellular function. While alterations Supplementation in NAD homeostasis are a common feature of different conditions and age-associated diseases, in general, in- creased NAD levels have been associated with beneficial health effects. Due to its therapeutic potential, the interest in this molecule has been renewed, and the regulation of NAD metabolism has become an attractive target for drug discovery. In fact, different approaches to replenish or increase NAD levels have been tested, including enhancement of biosynthesis and inhibition of NAD breakdown. Despite further research is needed, this review provides an overview and update on NAD metabolism, including the therapeutic potential of its regulation, as well as pharmacokinetics, safety, precautions and formulation challenges of NAD supplementa- tion. -

Metabolic-Hydroxy and Carboxy Functionalization of Alkyl Moieties in Drug Molecules: Prediction of Structure Influence and Pharmacologic Activity
molecules Review Metabolic-Hydroxy and Carboxy Functionalization of Alkyl Moieties in Drug Molecules: Prediction of Structure Influence and Pharmacologic Activity Babiker M. El-Haj 1,* and Samrein B.M. Ahmed 2 1 Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences, University of Science and Technology of Fujairah, Fufairah 00971, UAE 2 College of Medicine, Sharjah Institute for Medical Research, University of Sharjah, Sharjah 00971, UAE; [email protected] * Correspondence: [email protected] Received: 6 February 2020; Accepted: 7 April 2020; Published: 22 April 2020 Abstract: Alkyl moieties—open chain or cyclic, linear, or branched—are common in drug molecules. The hydrophobicity of alkyl moieties in drug molecules is modified by metabolic hydroxy functionalization via free-radical intermediates to give primary, secondary, or tertiary alcohols depending on the class of the substrate carbon. The hydroxymethyl groups resulting from the functionalization of methyl groups are mostly oxidized further to carboxyl groups to give carboxy metabolites. As observed from the surveyed cases in this review, hydroxy functionalization leads to loss, attenuation, or retention of pharmacologic activity with respect to the parent drug. On the other hand, carboxy functionalization leads to a loss of activity with the exception of only a few cases in which activity is retained. The exceptions are those groups in which the carboxy functionalization occurs at a position distant from a well-defined primary pharmacophore. Some hydroxy metabolites, which are equiactive with their parent drugs, have been developed into ester prodrugs while carboxy metabolites, which are equiactive to their parent drugs, have been developed into drugs as per se. -

The Stimulants and Hallucinogens Under Consideration: a Brief Overview of Their Chemistry and Pharmacology
Drug and Alcohol Dependence, 17 (1986) 107-118 107 Elsevier Scientific Publishers Ireland Ltd. THE STIMULANTS AND HALLUCINOGENS UNDER CONSIDERATION: A BRIEF OVERVIEW OF THEIR CHEMISTRY AND PHARMACOLOGY LOUIS S. HARRIS Dcparlmcnl of Pharmacology, Medical College of Virginia, Virginia Commonwealth Unwersity, Richmond, VA 23298 (U.S.A.) SUMMARY The substances under review are a heterogenous set of compounds from a pharmacological point of view, though many have a common phenylethyl- amine structure. Variations in structure lead to marked changes in potency and characteristic action. The introductory material presented here is meant to provide a set of chemical and pharmacological highlights of the 28 substances under con- sideration. The most commonly used names or INN names, Chemical Abstract (CA) names and numbers, and elemental formulae are provided in the accompanying figures. This provides both some basic information on the substances and a starting point for the more detailed information that follows in the individual papers by contributors to the symposium. Key words: Stimulants, their chemistry and pharmacology - Hallucinogens, their chemistry and pharmacology INTRODUCTION Cathine (Fig. 1) is one of the active principles of khat (Catha edulis). The structure has two asymmetric centers and exists as two geometric isomers, each of which has been resolved into its optical isomers. In the plant it exists as d-nor-pseudoephedrine. It is a typical sympathomimetic amine with a strong component of amphetamine-like activity. The racemic mixture is known generically in this country and others as phenylpropanolamine (dl- norephedrine). It is widely available as an over-the-counter (OTC) anti- appetite agent and nasal decongestant. -

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

Toxicology Times
TOXICOLOGY (800) 677-7995 TIMES www.sdrl.com A FREE Monthly Newsletter for Substance Abuse and Opioid Treatment Volume 5, Issue 7 Programs from San Diego Reference Laboratory July, 2015 Amphetamines (Part 1) Dr. Joseph E. Graas, Scientific Director most often used in inhalers and does not tite, increased stamina and physical energy, Dr. Edward Moore, Medical Director have the central nervous system activity nor increased sexual response/drive, involuntary any addictive properties. All of the com- body movements, increased perspiration, The term “amphetamines” has come to mean pounds that have the structure of hyperactivity, nausea and increased heart rate. a class of endogenous neurotransmitters that phenylethylamine will have this mixture of d This drug is highly addictive and tolerance stimulate the sympathetic nervous system. and l molecules. In the production of these develops quickly. Withdrawal is an extremely This is a broad class of various substituted drugs they are usually noted as a racemic unpleasant experience. A few street names derivatives of phenylethylamine. This grow- mixture, or specifically, as the d or l com- for the drug are amp, speed, crank, dolls and ing class of structurally related molecules may pound. To properly evaluate this family of crystal. also stimulate the sympathetic nervous sys- compounds known as sympathomimetic tem in many different ways, such as affecting amines, amphetamines or phenylethylamine, Methamphetamine was developed by the the re-release of neurotransmitters or pre- it is best done by describing each member of Japanese in 1919 and used during World War venting the re-uptake of neurotransmitters, the class: amphetamine , methampheta- II to help soldiers stay alert and energize hallucinogens, anorectics, bronchodilators mine , phentermine , phenylpropanola- factory workers. -

Drugbank 3.0: a Comprehensive Resource for 'Omics' Research On
Published online 8 November 2010 Nucleic Acids Research, 2011, Vol. 39, Database issue D1035–D1041 doi:10.1093/nar/gkq1126 DrugBank 3.0: a comprehensive resource for ‘Omics’ research on drugs Craig Knox1, Vivian Law2, Timothy Jewison1, Philip Liu3, Son Ly2, Alex Frolkis1, Allison Pon1, Kelly Banco2, Christine Mak2, Vanessa Neveu1, Yannick Djoumbou3, Roman Eisner1, An Chi Guo1 and David S. Wishart1,2,3,4,* 1Department of Computing Science, University of Alberta, Edmonton, AB, Canada T6G 2E8, 2Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, AB, Canada T6G 2N8, 3Department of Biological Sciences, University of Alberta, Edmonton, AB, Canada T6G 2E8 and 4National Institute for Nanotechnology, 11421 Saskatchewan Drive, Edmonton, AB, Canada T6G 2M9 Received September 15, 2010; Revised October 20, 2010; Accepted October 21, 2010 ABSTRACT drug target, drug description and drug action data. DrugBank (http://www.drugbank.ca) is a richly DrugBank 3.0 represents the result of 2 years annotated database of drug and drug target infor- of manual annotation work aimed at making mation. It contains extensive data on the nomencla- the database much more useful for a wide ture, ontology, chemistry, structure, function, range of ‘omics’ (i.e. pharmacogenomic, action, pharmacology, pharmacokinetics, metabol- pharmacoproteomic, pharmacometabolomic and ism and pharmaceutical properties of both small even pharmacoeconomic) applications. molecule and large molecule (biotech) drugs. It also contains comprehensive information on the INTRODUCTION target diseases, proteins, genes and organisms on which these drugs act. First released in 2006, Historically most of the known information on drugs, DrugBank has become widely used by pharmacists, drug targets and drug action has resided in books, medicinal chemists, pharmaceutical researchers, journals and expensive commercial databases. -

Smart Drugs: a Review
International Journal for Innovation Education and Research www.ijier.net Vol:-8 No-11, 2020 Smart Drugs: A Review Sahjesh Soni, Dr Rashmi Srivastava, Ayush Bhandari Mumbai Educational Trust, India Abstracts Smart drugs can change the way our mind functions. Smart drugs are also known as nootropics, which literally means the ability to bend or shape our mind. Smart drugs are classified into two main categories. They are classified based on their pharmacological action and their availability. The stimulant category of drugs is highly used and misused. There has been a rampant increase in the sale of smart drugs, which could be attributed to the rise in competition all over the world. Two major criteria for selecting a good drug are its mechanism of action and bioavailability. Owing to the short-term benefits of smart drugs, many countries have openly accepted this concept. There is still no concrete scientific evidence backing the safety and efficacy of these drugs. Some believe that this is just a fad that will soon pass, while others believe that this is something that will revolutionize our future. Key Words: Smart drugs, Nootropics, Cognitive enhancers, Stimulants, Uses and Side effects. What are Smart Drugs? "Smart drugs" are a group of compounds that can promote brain performance. They have got a lot of attention due to our stressful lifestyle, and these drugs help to boost our memory, focus, creativity, intelligence, and motivation. The origin of the word comes from the Greek language meaning “to bend or shape the mind”.1 These chemicals have many mechanisms of action. -

Monoamine Reuptake Inhibitors in Parkinson's Disease
Hindawi Publishing Corporation Parkinson’s Disease Volume 2015, Article ID 609428, 71 pages http://dx.doi.org/10.1155/2015/609428 Review Article Monoamine Reuptake Inhibitors in Parkinson’s Disease Philippe Huot,1,2,3 Susan H. Fox,1,2 and Jonathan M. Brotchie1 1 Toronto Western Research Institute, Toronto Western Hospital, University Health Network, 399 Bathurst Street, Toronto, ON, Canada M5T 2S8 2Division of Neurology, Movement Disorder Clinic, Toronto Western Hospital, University Health Network, University of Toronto, 399BathurstStreet,Toronto,ON,CanadaM5T2S8 3Department of Pharmacology and Division of Neurology, Faculty of Medicine, UniversitedeMontr´ eal´ and Centre Hospitalier de l’UniversitedeMontr´ eal,´ Montreal,´ QC, Canada Correspondence should be addressed to Jonathan M. Brotchie; [email protected] Received 19 September 2014; Accepted 26 December 2014 Academic Editor: Maral M. Mouradian Copyright © 2015 Philippe Huot et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The motor manifestations of Parkinson’s disease (PD) are secondary to a dopamine deficiency in the striatum. However, the degenerative process in PD is not limited to the dopaminergic system and also affects serotonergic and noradrenergic neurons. Because they can increase monoamine levels throughout the brain, monoamine reuptake inhibitors (MAUIs) represent potential therapeutic agents in PD. However, they are seldom used in clinical practice other than as antidepressants and wake-promoting agents. This review article summarises all of the available literature on use of 50 MAUIs in PD. The compounds are divided according to their relative potency for each of the monoamine transporters. -

Whoexpertcommittee Ondrugdependence
Thisreportcontainsthecollectiveviewsof an international groupof expertsanddoesnotnecessarilyrepresentthedecisions Tile World 1lealth Organization is a specialized agcncyof the United Nations with orthestatedpolicyof theWorldHealthOrganization primary responsibility for international health'matters anti public health. Through this organization, which was created in 1948, the health professions of' some I65 countries exchange their knowledge and experience with the aim of making possible will permit them lo lead a socially and ccommlically productive lil_. WHOExpertCommittee By means of direct technical cooperation whh its Membcr Stales, and by stimt,- hhensiveding suchhealthcooperationservices, tamonghe preventionthem, WllOprornotesthcdevclopmcntorcomprc-anti control of diseascs, thc improvement o[ environmental conditions, tile development of health manpower, the coordination onDrugDependence anti development of biomedical and health services research, and thc planning and implementation of health programmes. These broad fiekls of endeavour encompass a wide variety of activities, such as developing systems of primary health cltre that reach the whole population of Mem- ber countries; promoting tile health of`mothers and children; combating malnutrition; controlling malaria and other communicable diseases including tuberculosis and leprosy; having achieved tile eradication or smallpox, promoting mass immunization against a numbcr of other preventable diseases; improving mental health; providing safe water supplies: anti training health -

Drug Knowledge Bases and Their Applications in Biomedical Informatics Research Yongjun Zhu, Olivier Elemento, Jyotishman Pathak and Fei Wang
Briefings in Bioinformatics, 2018, 1–14 doi: 10.1093/bib/bbx169 Paper Drug knowledge bases and their applications in biomedical informatics research Yongjun Zhu, Olivier Elemento, Jyotishman Pathak and Fei Wang Corresponding author: Fei Wang, Division of Health Informatics, Department of Healthcare Policy and Research at Weill Cornell Medicine at Cornell University, 425 East 61st Street, Suite 301, DV-308, New York, NY 10065, USA. E-mail: [email protected] Abstract Recent advances in biomedical research have generated a large volume of drug-related data. To effectively handle this flood of data, many initiatives have been taken to help researchers make good use of them. As the results of these initiatives, many drug knowledge bases have been constructed. They range from simple ones with specific focuses to comprehensive ones that contain information on almost every aspect of a drug. These curated drug knowledge bases have made significant contributions to the development of efficient and effective health information technologies for better health-care service delivery. Understanding and comparing existing drug knowledge bases and how they are applied in various biomedical studies will help us recognize the state of the art and design better knowledge bases in the future. In addition, researchers can get insights on novel applications of the drug knowledge bases through a review of successful use cases. In this study, we provide a review of existing popular drug knowledge bases and their applications in drug-related studies. We discuss challenges in constructing and using drug knowledge bases as well as future research directions toward a better ecosystem of drug knowledge bases. -
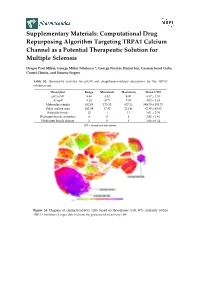
Computational Drug Repurposing Algorithm Targeting TRPA1 Calcium Channel As a Potential Therapeutic Solution for Multiple Sclerosis
Supplementary Materials: Computational Drug Repurposing Algorithm Targeting TRPA1 Calcium Channel as a Potential Therapeutic Solution for Multiple Sclerosis Dragos Paul Mihai, George Mihai Nitulescu *, George Nicolae Daniel Ion, Cosmin Ionut Ciotu, Cornel Chirita, and Simona Negres Table S1. Descriptive statistics for pIC50 and druglikeness-related descriptors for the TRPA1 inhibitors set. Descriptor Range Minimum Maximum Mean ± SD pIC50 (M) 4.48 4.52 9.00 6.57 ± 1.01 ALogP 8.28 −0.71 7.57 4.02 ± 1.34 Molecular weight 482.03 175.10 657.13 389.70 ± 101.73 Polar surface area 193.59 17.82 211.41 82.80 ± 40.83 Rotatable bonds 12 1 13 5.01 ± 2.08 Hydrogen bonds acceptors 8 0 8 2.92 ± 1.46 Hydrogen bonds donors 3 0 3 1.06 ± 0.54 SD – standard deviation. Figure S1. Diagram of similarity/activity cliffs based on flexophores with 80% similarity within TRPA1 inhibitors. Larger dots indicate the presence of an activity cliff. Figure S2. Representative structures for similarity/activity cliffs analysis of TRPA1 inhibitors. Table S2. Highest similarity pairs between TRPA1 inhibitors and screened drugs based on flexophore descriptors data mining procedure. TRPA1 inhibitors Repurposing dataset Entry Similarity (ChEMBL ID) (DrugBank ID) 1 CHEMBL3298238 DB08135 0.9832 2 CHEMBL3220230 DB08561 0.9696 3 CHEMBL3220228 DB08561 0.9614 4 CHEMBL593902 DB07311 0.9553 5 CHEMBL3297780 DB01065 0.9533 6 CHEMBL3220448 DB08561 0.9509 Figure S3. Diagram of similarity/activity cliffs based on flexophores with 80% similarity threshold for merged TRPA1 inhibitors dataset (colored dots) and similar DrugBank entries (grey dots). Table S3.