Dietary Supplement Ingredient Database (DSID): Preliminary USDA Studies on the Composition of Adult Multivitamin/Mineral Supplements$
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Complete Course
A Complete Course Forum Theological Midwest Author: Rev.© Peter V. Armenio Publisher:www.theologicalforum.org Rev. James Socias Copyright MIDWEST THEOLOGICAL FORUM Downers Grove, Illinois iii CONTENTS xiv Abbreviations Used for 43 Sidebar: The Sanhedrin the Books of the Bible 44 St. Paul xiv Abbreviations Used for 44 The Conversion of St. Paul Documents of the Magisterium 46 An Interlude—the Conversion of Cornelius and the Commencement of the Mission xv Foreword by Francis Cardinal George, to the Gentiles Archbishop of Chicago 47 St. Paul, “Apostle of the Gentiles” xvi Introduction 48 Sidebar and Maps: The Travels of St. Paul 50 The Council of Jerusalem (A.D. 49– 50) 1 Background to Church History: 51 Missionary Activities of the Apostles The Roman World 54 Sidebar: Magicians and Imposter Apostles 3 Part I: The Hellenistic Worldview 54 Conclusion 4 Map: Alexander’s Empire 55 Study Guide 5 Part II: The Romans 6 Map: The Roman Empire 59 Chapter 2: The Early Christians 8 Roman Expansion and the Rise of the Empire 62 Part I: Beliefs and Practices: The Spiritual 9 Sidebar: Spartacus, Leader of a Slave Revolt Life of the Early Christians 10 The Roman Empire: The Reign of Augustus 63 Baptism 11 Sidebar: All Roads Lead to Rome 65 Agape and the Eucharist 12 Cultural Impact of the Romans 66 Churches 13 Religion in the Roman Republic and 67 Sidebar: The Catacombs Roman Empire 68 Maps: The Early Growth of Christianity 14 Foreign Cults 70 Holy Days 15 Stoicism 70 Sidebar: Christian Symbols 15 Economic and Social Stratification of 71 The Papacy Roman -

Western Europe After the Fall of Rome
1/17/2012 Effects of Germanic Invasions End of the 5th Century Raiders, • Repeated invasions, constant warfare Traders and – Disruption of trade • Merchants invaded from land and sea— Crusaders: businesses collapse – Downfall of cities Western Europe • Cities abandoned as centers for administration After the Fall of – Population shifts • Nobles and other city-dwellers retreat to rural Rome areas • Population of Western Europe became mostly rural The Rise of Europe 500-1300 The Early Middle Ages • Europe was cut off from the advanced civilizations of Byzantium, the Middle East, China and India. • Between 700 and 1000, Europe was battered by invaders. • New forms of social organization developed amid the fragmentation • Slowly a new civilization would emerge that blended Greco-Roman, Germanic and Christian traditions. akupara.deviantart.com Guiding Questions Decline of Learning 1.How was Christianity a unifying social and • Germanic invaders could not read or write political factor in medieval Europe? – Oral tradition of songs and legends • Literacy dropped among those moved to rural areas 2.What are the characteristics of Roman • Priests and other church officials were among Catholicism? the few who were literate • Greek Knowledge was almost lost – Few people could read Greek works of literature, science, and philosophy 1 1/17/2012 Loss of Common Language A European Empire Evolves • Latin changes as German-speaking • After the Roman Empire, Europe divided into people mix with Romans 7 small kingdoms (some as small as Conn.) • No longer understood from region to • The Christian king Clovis ruled the Franks region (formerly Gual) • By 800s, French, Spanish, and other • When he died in 511, the kingdom covered Roman-based (Romance) languages much of what is now France. -

Increasing Egg Consumption at Breakfast Is Associated With
nutrients Article Increasing Egg Consumption at Breakfast Is Associated with Increased Usual Nutrient Intakes: A Modeling Analysis Using NHANES and the USDA Child and Adult Care Food Program School Breakfast Guidelines Yanni Papanikolaou 1,* and Victor L. Fulgoni III 2 1 Nutritional Strategies, Nutrition Research & Regulatory Affairs, 59 Marriott Place, Paris, ON N3L 0A3, Canada 2 Nutrition Impact, Nutrition Research, 9725 D Drive North, Battle Creek, MI 49014, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-519-504-9252 Abstract: The objective of the current modeling analysis was three-fold: (1) to examine usual nutrient intakes in children when eggs are added into dietary patterns that typically do not contain eggs; (2) to examine usual nutrient intakes with the addition of eggs in the Child and Adult Care Food Program (CACFP) school breakfast; and (3) to examine nutrient adequacy when eggs are included in routine breakfast patterns and with the addition of eggs to the CACFP school breakfast program. Dietary recall data from the National Health and Nutrition Examination Survey 2011–2016 (children aged 1–18 years-old; n = 9254; CACFP n = 159) were used in the analysis. The usual intakes of pantothenic acid, riboflavin, selenium, and vitamin D increased ≥10 percent (relative to the baseline values) with Citation: Papanikolaou, Y.; Fulgoni, the addition of one egg at breakfast. The usual intakes of protein and vitamin A at breakfast were V.L., III Increasing Egg Consumption also increased by more than 10 percent compared to the baseline values with the addition of two at Breakfast Is Associated with eggs. -

University of Birmingham Algerian Women and the Traumatic
University of Birmingham Algerian Women and the Traumatic Decade: Daoudi, Anissa License: None: All rights reserved Document Version Peer reviewed version Citation for published version (Harvard): Daoudi, A 2017, 'Algerian Women and the Traumatic Decade: Literary Interventions', Journal of Literature and Trauma Studies. Link to publication on Research at Birmingham portal General rights Unless a licence is specified above, all rights (including copyright and moral rights) in this document are retained by the authors and/or the copyright holders. The express permission of the copyright holder must be obtained for any use of this material other than for purposes permitted by law. •Users may freely distribute the URL that is used to identify this publication. •Users may download and/or print one copy of the publication from the University of Birmingham research portal for the purpose of private study or non-commercial research. •User may use extracts from the document in line with the concept of ‘fair dealing’ under the Copyright, Designs and Patents Act 1988 (?) •Users may not further distribute the material nor use it for the purposes of commercial gain. Where a licence is displayed above, please note the terms and conditions of the licence govern your use of this document. When citing, please reference the published version. Take down policy While the University of Birmingham exercises care and attention in making items available there are rare occasions when an item has been uploaded in error or has been deemed to be commercially or otherwise sensitive. If you believe that this is the case for this document, please contact [email protected] providing details and we will remove access to the work immediately and investigate. -

The Early Middle Ages
The Early Middle Ages After the collapse of Rome, Western Europe entered a period of political, social, and economic decline. From about 500 to 1000, invaders swept across the region, trade declined, towns emptied, and classical learning halted. For those reasons, this period in Europe is sometimes called the “Dark Ages.” However, Greco-Roman, Germanic, and Christian traditions eventually blended, creating the medieval civilization. This period between ancient times and modern times – from about 500 to 1500 – is called the Middle Ages. The Frankish Kingdom The Germanic tribes that conquered parts of the Roman Empire included the Goths, Vandals, Saxons, and Franks. In 486, Clovis, king of the Franks, conquered the former Roman province of Gaul, which later became France. He ruled his land according to Frankish custom, but also preserved much of the Roman legacy by converting to Christianity. In the 600s, Islamic armies swept across North Africa and into Spain, threatening the Frankish kingdom and Christianity. At the battle of Tours in 732, Charles Martel led the Frankish army in a victory over Muslim forces, stopping them from invading France and pushing farther into Europe. This victory marked Spain as the furthest extent of Muslim civilization and strengthened the Frankish kingdom. Charlemagne After Charlemagne died in 814, his heirs battled for control of the In 786, the grandson of Charles Martel became king of the Franks. He briefly united Western empire, finally dividing it into Europe when he built an empire reaching across what is now France, Germany, and part of three regions with the Treaty of Italy. -

Unit 5: the Post-Classical Period: the First Global Civilizations
Unit 5: The Post-Classical Period: The First Global Civilizations Name: ________________________________________ Teacher: _____________________________ IB/AP World History 9 Commack High School Please Note: You are responsible for all information in this packet, supplemental handouts provided in class as well as your homework, class webpage and class discussions. What do we know about Muhammad and early Muslims? How do we know what we know? How is our knowledge limited? Objective: Evaluate the primary sources that historians use to learn about early Muslims. Directions: Below, write down two things you know about Muhammad and how you know these things. What I know about Muhammad... How do I know this …. / Where did this information come from... Directions: Below, write down two things you know about Muslims and how you know these things. What I know about Muslims... How do I know this …. / Where did this information from from... ARAB EXPANSION AND THE ISLAMIC WORLD, A.D. 570-800 1. MAKING THE MAP 1. Locate and label: 4. Locate and label: a Mediterranean Sea a Arabian Peninsula b Atlantic Ocean b Egypt c Black Sea c Persia (Iran) d Arabian Sea d Anatolia e Caspian Sea e Afghanistan f Aral Sea f Baluchistan g Red Sea g Iraq h Persian Gulf. 2. Locate and label: h Syria a Indus River i Spain. b Danube River 5. Locate and label: c Tigris River a Crete b Sicily d Euphrates River c Cyprus e Nile River d Strait of Gibraltar f Loire River. e Bosphorus. 3. Locate and label: 6. Locate with a black dot and a Zagros Mountains label: b Atlas Mountains a Mecca c Pyrenees Mountains b Medina d Caucasus Mountains c Constantinople e Sahara Desert. -
Chapter 4: China in the Middle Ages
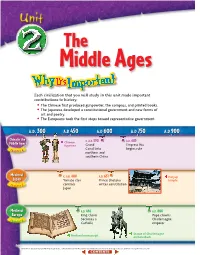
The Middle Ages Each civilization that you will study in this unit made important contributions to history. • The Chinese first produced gunpowder, the compass, and printed books. • The Japanese developed a constitutional government and new forms of art and poetry. • The Europeans took the first steps toward representative government. A..D.. 300300 A..D 450 A..D 600 A..D 750 A..DD 900 China in the c. A.D. 590 A.D.683 Middle Ages Chinese Middle Ages figurines Grand Empress Wu Canal links begins rule Ch 4 apter northern and southern China Medieval c. A.D. 400 A.D.631 Horyuji JapanJapan Yamato clan Prince Shotoku temple Chapter 5 controls writes constitution Japan Medieval A.D. 496 A.D. 800 Europe King Clovis Pope crowns becomes a Charlemagne Ch 6 apter Catholic emperor Statue of Charlemagne Medieval manuscript on horseback 244 (tl)The British Museum/Topham-HIP/The Image Works, (c)Angelo Hornak/CORBIS, (bl)Ronald Sheridan/Ancient Art & Architecture Collection, (br)Erich Lessing/Art Resource, NY 0 60E 120E 180E tecture Collection, (bl)Ron tecture Chapter Chapter 6 Chapter 60N 6 4 5 0 1,000 mi. 0 1,000 km Mercator projection EUROPE Caspian Sea ASIA Black Sea e H T g N i an g Hu JAPAN r i Eu s Ind p R Persian u h . s CHINA r R WE a t Gulf . e PACIFIC s ng R ha Jiang . C OCEAN S le i South N Arabian Bay of China Red Sea Bengal Sea Sea EQUATOR 0 Chapter 4 ATLANTIC Chapter 5 OCEAN INDIAN Chapter 6 OCEAN Dahlquist/SuperStock, (br)akg-images (tl)Aldona Sabalis/Photo Researchers, (tc)National Museum of Taipei, (tr)Werner Forman/Art Resource, NY, (c)Ancient Art & Archi NY, Forman/Art Resource, (tr)Werner (tc)National Museum of Taipei, (tl)Aldona Sabalis/Photo Researchers, A..D 1050 A..D 1200 A..D 1350 A..D 1500 c. -

General Instructions Income Tax
GENERAL INSTRUCTIONS INCOME TAX INTRODUCTION must be the same as the Federal. A copy of the Federal return The Georgia law recognizes an election to file as an S Corpo- and all supporting schedules must be attached to the Georgia ration under the provisions of the IRC as it existed on January return. If a Federal audit results in a change in taxable income, 1, 2002, qualified only in cases of nonresident shareholders, the taxpayer shall make a return to the Commissioner of the who must complete Form 600 S-CA (see Page 9). It also pro- changed or corrected net income within 180 days of final deter- vides for the imposition of a Net Worth Tax. mination. The return should be mailed to: Georgia Income Tax Division, P.O. Box 49432, Atlanta, Georgia 30359-1432. FILING REQUIREMENTS COMPUTING GEORGIA TAXABLE INCOME All corporations owning property or doing business within SCHEDULE 1 Georgia are required to file a Georgia income tax return. (Please If an S Corporation is required to pay a tax at the federal level, round all dollar entries.) A corporation electing the provisions of it may be required to pay a tax at the state level. This schedule the IRC for S Corporations, having one or more stockholders applies only to S Corporations which have converted from a C who are nonresidents of Georgia, must file a Form 600 S-CA Corporation and are subject to the corporate income tax due to on behalf of each nonresident. Failure to furnish a properly Excess Net Passive Investment Income, Capital Gains or Built executed Form 600 S-CA for each nonresident stockholder in Capital Gains. -

7.1 Introduction Our Study of Islam Begins with the Arabian Peninsula
Name and Date: _________________________ Text: HISTORY ALIVE! The Medieval World 7.1 Introduction Our study of Islam begins with the Arabian Peninsula, where Islam was first preached. The founder of Islam, Muhammad, was born on the peninsula in about 570 C.E. In this chapter, you’ll learn about the peninsula’s geography and the ways of life of its people in the sixth century. The Arabian Peninsula is in southwest Asia, between the Red Sea and the Persian Gulf. It is often called Arabia. Along with North Africa, the eastern Mediterranean shore, and present day Turkey, Iraq, and Iran, it is part of the modern Middle East. Most of the people living in Arabia in the sixth century were Arabs. Some Arabs call their home al-Jazeera, or “the Island.” But it is surrounded by water on only three sides. The Persian Gulf lies to the east, the Red Sea to the west, and the Indian Ocean to the south. To the north are lands bordering the Mediterranean Sea. These lands serve as a land bridge between Africa, Asia, and Europe. Imagine that you are flying over the Arabian Peninsula. As you look down, you see vast deserts dotted by oases. Coastal plains line the southern and western coasts. Mountain ranges divide these coastal plains from the desert. The hot, dry Arabian Peninsula is a challenging place to live. In this chapter, you will study the geography of Arabia and its different environments. You’ll see how people made adaptations in order to thrive there. 7.2 The Importance of the Arabian Peninsula and Surrounding Lands Arabia lies at the crossroads of Asia, Africa, and Europe. -

What Led to the Tang (618–906) and Song (960–1279) Dynasty Golden Ages? ➡ Directions: Examine the Text and Map Below, Then Respond to the Questions
Name ______________________________________ Per. ______ Pg. # ______ What led to the Tang (618–906) and Song (960–1279) Dynasty Golden Ages? ➡ Directions: Examine the text and map below, then respond to the questions. Contextualize During the Tang (618–906) and Song (960–1279) Dynasties, China was one of the most technologically advanced civilizations in the world. Innovation in art, science, philosophy, and technology gave the people in these societies an understanding of the world around them that other civilizations and even later dynasties in China would not accomplish for hundreds of years. Like other golden ages in history, the Tang and Song Dynasties unified the region, created a strong central government, and ruled over a time of peace that was reinforced by the government’s actions. The Tang expanded China’s territories and influence to include Korea and Vietnam, as well as portions of northeast, central, and southeast Asia. The peace that the Tang and Song governments created encouraged trade throughout the empire and abroad through the Silk Roads. Chinese cities during the Tang and Song dynasties became prosperous cultural centers of an international age where religious, academic, and artistic life and cultural exchange flourished. Scholars and merchants from as far as Persia and India came to Tang China to participate in its golden age. While the government was strong, it was not oppressive. Some became very rich, but the Tang rulers redistributed land to give the less fortunate an opportunity to grow in wealth. In addition this was a time when Source: Goldberg and DuPré, Brief Review in Global History and Geography, Prentice Hall (adapted) from the NYS education and achievement were rewarded and women attained a higher Global History and Geography Regents Exam, June 2005. -

Butterfly Valves
STANDARD SPECIFICATION � 軽荷重形バタフライバルブ� LIGHT DUTY DAMPER TYPE BUTTERFLY VALVES 600S SERIES Title:210E表2.ec5 Page:2 の自動調節弁と計装システム は、自動調節弁(コントロールバルブ)のトップメーカとして高水準にある独自の技術開 発力で時代の要請に応え、新世紀へ対応する幅広い製品を揃えるとともに、ISO 9001による品質保 証体制のもとで製作した高品質、高信頼のコントロールバルブとそのシステムを提供してまいりま す。ここに紹介する資料は、 コントロールバルブの技術カタログであります。 コントロールバルブの選定に御利用下さい。更に詳細な資料が御必要な場合又は、本技術カタログ について不明な点がございましたら最寄りの弊社営業までお問い合わせ下さい。 , the leading industrial control valve manufacturer with strong research and de- velopment capability of its own, has been meeting requirements of the time. Always making available a wide range of product lines that can satisfy the needs of the coming century, is committed to providing control valves, and the systems thereof, of highest quality and reliability, produced under its quality assurance system complying with ISO 9001 standard. Here presents its technological catalog, introducing various types of control valves, hoping to be of service as you select valves for your specific applications. If you have questions on this technological catalog or require additional printed materials, please contact our sales representative nearest you. Title:600本文.ec5 Page:1 STANDARD SPECIFICATION 600S Light Duty Damper Type Butterfly Valves 概 要 このシリーズの弁本体及びディスクは、鋼板を加工し The valve body and the disc of the 600S Series Butterfly て製作しますので、軽量で構造も簡単なバタフライバ Valves are made of machined steel plates. This allows light ルブです。主な用途としては、低圧の空気、ガスなど and simple constraction. Typical applications are flow/ の流量、圧力制御に適しています。又、低圧の高温流 pressure controls of low-pressure air and gas. High 体への対応も比較的安価で容易にできます。 temperature fluid at low pressure levels can also be accommodated inexpensively and easily. 標準仕様 弁本体部 シリーズ Series 600S 3″~60″ (80A~1500A) 弁サイズ Body size 最大製作実績 The largest size manufactured: 160″(4000mm) ダンパー形 (図2-1) Damper type (See Fig. 2-1) ディスク形状 Disc form 15度密閉形 (図2-2) 15 deg Sealed type (See Fig. -

Dawson Period Coverage
1 Dawson Period Coverage “Cycles of Civilisation” (1922) Progress and Religion Understanding Europe (Chapter 2) The Historic Reality of Christian Culture (Chapter 3) Age I [4500BC-2700BC]—Egypt Chap. 5 Religion and the Origins of Chapter II. “Europe and the Seven and Babylonia Civilization Stages of Western Culture” which are: Age II [2700-1100BC]—Later Egypt and the Aegean Age III [1200BC-800AD] Growth: 1200-900BC—Decline of Age II and growth of new ideas 900-500BC—Flourishing of Civic Religion-Culture Progress: ca. 6th- 5th century BC—Intellectual Awakening [Axial Age] 4th to 1st century BC—Expansion of the West (Roman Republic, Great periods in China] 1. Hellenism—the paideia Maturity: 1st century BC to 3rd 2. The Roman World—the Pax century AD—Rise of World Romana and canon law Age 1: From Pentecost to Religions— Buddhism, Chap. 6 The Rise of the World Constantine (AD 325) Christianity, Taoism, Mazdean in Religions Persia rd th 3 to 7 century AD— Flourishing of Religion- 2 Cultures—Gupta India, Persia, 3. Formation of Western and Period and North and South in Eastern Christendom—the Age 2: Christian Empire in the China, early Byzantine Chap. 7 Christianity and the Rise of patristic era, monasticism, the West (325-600s) Western Civilization Carolingian Renaissance Age IV [500-1920AD] Age 3: Islamic and Viking Growth (The Middle Ages): Invasions (7th century to apprx. 500-750AD: Fertilization of 1000 AD) daughter cultures: India, China, Islam 9th and 12th centuries: Flourishing of daughter cultures: Carolingian, Japan, the Age 4: Medieval Christendom th th th Caliphate, Sankara and 4.