From Roraima, Brazil: Species Composition, Distribution, and New Records
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two Freshwater Shrimp Species of the Genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the Description of a New Species
A peer-reviewed open-access journal ZooKeys 923: 15–32 (2020) Caridina tetrazona 15 doi: 10.3897/zookeys.923.48593 RESEarcH articLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species Qing-Hua Chen1, Wen-Jian Chen2, Xiao-Zhuang Zheng2, Zhao-Liang Guo2 1 South China Institute of Environmental Sciences, Ministry of Ecology and Environment, Guangzhou 510520, Guangdong Province, China 2 Department of Animal Science, School of Life Science and Enginee- ring, Foshan University, Foshan 528231, Guangdong Province, China Corresponding author: Zhao-Liang Guo ([email protected]) Academic editor: I.S. Wehrtmann | Received 19 November 2019 | Accepted 7 February 2020 | Published 1 April 2020 http://zoobank.org/138A88CC-DF41-437A-BA1A-CB93E3E36D62 Citation: Chen Q-H, Chen W-J, Zheng X-Z, Guo Z-L (2020) Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species. ZooKeys 923: 15–32. https://doi.org/10.3897/zookeys.923.48593 Abstract A faunistic and ecological survey was conducted to document the diversity of freshwater atyid shrimps of Dawanshan Island. Two species of Caridina that occur on this island were documented and discussed. One of these, Caridina tetrazona sp. nov. is described and illustrated as new to science. It can be easily distinguished from its congeners based on a combination of characters, which includes a short rostrum, the shape of the endopod of the male first pleopod, the segmental ratios of antennular peduncle and third maxilliped, the slender scaphocerite, and the absence of a median projection on the posterior margin. -

Cerrado Protected Areas: Chapada Dos Veadeiros and Emas National Parks - 2017 Conservation Outlook Assessment (Archived)
IUCN World Heritage Outlook: https://worldheritageoutlook.iucn.org/ Cerrado Protected Areas: Chapada dos Veadeiros and Emas National Parks - 2017 Conservation Outlook Assessment (archived) IUCN Conservation Outlook Assessment 2017 (archived) Finalised on 12 November 2017 Please note: this is an archived Conservation Outlook Assessment for Cerrado Protected Areas: Chapada dos Veadeiros and Emas National Parks. To access the most up-to-date Conservation Outlook Assessment for this site, please visit https://www.worldheritageoutlook.iucn.org. Cerrado Protected Areas: Chapada dos Veadeiros and Emas National Parks SITE INFORMATION Country: Brazil Inscribed in: 2001 Criteria: (ix) (x) Site description: The two sites included in the designation contain flora and fauna and key habitats that characterize the Cerrado – one of the world’s oldest and most diverse tropical ecosystems. For millennia, these sites have served as refuge for several species during periods of climate change and will be vital for maintaining the biodiversity of the Cerrado region during future climate fluctuations. © UNESCO IUCN World Heritage Outlook: https://worldheritageoutlook.iucn.org/ Cerrado Protected Areas: Chapada dos Veadeiros and Emas National Parks - 2017 Conservation Outlook Assessment (archived) SUMMARY 2017 Conservation Outlook Good with some concerns The current state of conservation of the site is relatively good. Existing threats to ecological processes, biodiversity, threatened species, and other species of particular conservation concern are minor, and management programs are relatively effective. However, conservation of the site was seriously impacted when 72% of the CdVNP was excised from the Park until June 2017. Efforts have been undertaken to restore protection regime for most parts of the site, adding otherwise new areas. -

Conservation Versus Development at the Iguacu National Park, Brazil1
CONSERVATION VERSUS DEVELOPMENT AT THE IGUACU NATIONAL PARK, BRAZIL1 Ramon Arigoni Ortiz a a Research Professor at BC3 – Basque Centre for Climate Change – Bilbao – Spain Alameda Urquijo, 4 Piso 4 – 48009 – [email protected] Abstract The Iguacu National Park is a conservation unit that protects the largest remnant area of the Atlantic Rainforest in Brazil. The Colono Road is 17.6 km long road crossing the Iguacu National Park that has been the motive of dispute between environmentalists, government bodies and NGOs defending the closure of the Colono Road; and organised civil institutions representing the population of the surrounding cities defending its opening. In October 2003, 300 people invaded the Park in an attempt to remove the vegetation and reopen the road, which was prevented by members of the Brazilian Army and Federal Police. Those who advocate the reopening of the Colono Road claim significant economic losses imposed on the surrounding cities. This paper investigates this claim and concludes that a possible reopening of the Colono Road cannot be justified from an economic perspective. Keywords: Iguacu Park; Brazil; Colono Road; economic development; environmental degradation; valuation; cost-benefit analysis 1 WWF-Brazil provided the financial support to this work, which I am grateful. However, WWF-Brazil is not responsible for the results and opinions in this study. I am also grateful to two anonymous referees for their constructive comments, corrections and suggestions. The remaining errors and omissions are responsibility of the author solely. Ambientalia vol. 1 (2009-2010) 141-160 1 Arigoni, R. 1. INTRODUCTION sentence. The Colono Road remained closed until The Iguacu National Park is a conservation May 1997 when an entity named ´Friends of the unit located in Parana State, south region of Brazil Park´ (Movimento de Amigos do Parque) (Figure 1), comprising an area of 185,000 ha. -

Guia De Cursos Do IFRR
GUIA DE CURSOS TÉCNICOS LICENCIATURAS TECNOLOGIAS Ademar de Araújo Filho Reitor Ivone Mary Medeiros de Souza Pró-Reitora de Ensino Edvaldo Pereira da Silva Pró-Reitor de Extensão Jaci Lima da Silva Pró-Reitor de Pesquisa, Pós-Graduação e Inovação Tecnológica Carlos Roberto Cabral de Lima Pró-Reitor de Desenvolvimento Instucional Maria do Perpétuo Socorro Pereira Silva Pró-Reitora de Administração Milton José Piovesan Diretor Geral do Câmpus Boa Vista Eliezer Nunes da Silva Diretor Geral do Câmpus Novo Paraíso George Sterfson Barros Diretor Geral do Câmpus Amajari Maria Aparecida Alves de Medeiros Diretora Geral do Câmpus Zona Oeste de Boa Vista Arnóbio Gustavo Queiroz de Magalhães Diretor Geral do Câmpus Avançado do Bonfim Palavras do Reitor O Instuto Federal de Educação, Ciência e Tecnologia de Roraima - IFRR inaugura uma nova fase, após vivenciar as fases de Escola Técnica Federal de Roraima – ETFRR e Centro Federal de Educação Tecnológica de Roraima – CEFET/RR. Surge para consolidar o grande desafio e desejo de fortalecer e expandir o Ensino Profissional e Tecnológico no Estado de Roraima. Atualmente possui cinco Câmpus – Amajari, Boa Vista, Bonfim, Novo Paraíso e Zona Oeste de Boa Vista – cujas localizações estratégicas foram definidas de modo a atender os arranjos produvos locais. Além disso, atendem ao objevo principal do IFRR que é levar educação profissional e tecnológica de qualidade e de alto nível às localidades mais necessitadas, perpassando as diferentes modalidades e níveis de ensino, desde os cursos de Formação Inicial e Connuada – FIC, Formação Técnica de Nível Médio, Graduação até Pós-Graduação, sobretudo na área tecnológica. O IFRR visa à promoção da jusça social, da inclusão social e da equidade, para tanto, propõe-se a intervir significavamente numa proposta transformadora da educação profissional, afim de modificar a vida da sociedade onde está inserido, proporcionando novos conhecimentos cienficos e tecnológicos. -

Iucn Technical Evaluation Jaú
WORLD HERITAGE NOMINATION - IUCN TECHNICAL EVALUATION JAÚ NATIONAL PARK (EXTENSION TO FORM THE CENTRAL AMAZON PROTECTED AREAS) (BRAZIL) ID Nº 998 Bis 1. DOCUMENTATION (i) IUCN/WCMC Data Sheet: (10 references) (ii) Additional literature consulted: IUCN, 2000. IUCN Technical Evaluation, Jaú National Park (Brazil). SCM/CNPQ/MCT/IPAAM. 1996. Mamirauá: Plano de Manejo. Manaus: IPAAM. Queiroz, H., and M. E. B. Fernandes. 2001. A Regional Analysis of Geographic Priorities for Biodiversity Conservation in Latin America and the Caribbean. Washington, DC; Davis, S. D. et. al. Centres of Plant Diversity. Vol. 3. IUCN; Thorsell, J. and T. Sigaty, 1997. A global overview of forest protected areas on the World Heritage List (Draft). IUCN; Gillet, H. et. al., 1998. A global overview of protected areas on the World Heritage List of particular importance for biodiversity. UNESCO/WCMC/IUCN; Rylands, A. B., 1991. The status of conservation areas in the Brazilian Amazon. WWF, Washington DC; Rojas, M. and C. Castaño, 1990. Areas protegidas de la cuenca del Amazonas. Bogotá, Colombia ; Castaño. C., 1993. Situación general de la conservación de la biodiversidad en la región Amazónica: Evaluación de las áreas protegidas propuestas y estrategias. FAO/CEE/IUCN, Ecuador; Henrique Borges. S and Carvalhes, A., 2000. Bird species of black water inundation forest in the Jaú National Park: their contribution to regional species richness. In Biodiversity and Conservation, Vol. 9, No. 2, pp 201-214. (iii) Consultations: 5 external reviewers, representatives from the Ministry of Environment, Brazilian Institute of the Environment and Renewable Natural Resources (IBAMA), environmental authorities, from the States of Brasilia and Manaus, local staff of the protected areas from IBAMA and the Mamirauá Institute, and researchers from the University of Florida and the Amazon Research Institute (INPE). -

Divisão Territorial De Cuiabá
WILSON PEREIRA DOS SANTOS Prefeito Municipal de Cuiabá JACY RIBEIRO DE PROENÇA Vice Prefeita Municipal ANDELSON GIL DO AMARAL PEDRO PINTO DE OLIVEIRA ÉDEN CAPISTRANO PINTO JOÃO DE SOUZA VIEIRA FILHO Secretário Municipal de Governo Secretário Municipal de Secretário Municipal de Meio Secretário Municipal de Trabalho, Comunicação Ambiente e Desenvolvimento Urbano Desenv. Econômico e Turismo. JOSÉ ANTÔNIO ROSA GUILHERME FREDERICO MÜLLER OSCAR SOARES MARTINS ADRIANA BUSSIKI SANTOS Procurador Geral do Município Secretário Municipal de Secretário Municipal de Trânsito e Presidente do Instituto de Pesquisa Planejamento, Orçamento e Gestão. Transporte Urbano e Desenvolvimento Urbano MÁRIO OLÍMPIO MEDEIROS FILHO GUILHERME ANTÔNIO MALUF PEDRO LUIZ SHINOHARA JÚLIO CÉSAR PINHEIRO Secretário Municipal de Cultura Secretária Municipal de Saúde Secretário Municipal de Esporte e Presidente da Agência Municipal de Cidadania Habitação Popular CELCITA ROSA PINHEIRO DA SILVA JOSÉ CARLOS CARVALHO SOUZA EDUARDO ALEXANDRE RICCI RONALDO ROSA TAVEIRA Secretário Municipal de Assistência Secretário Municipal de Finanças Ouvidor Geral do Município de Presidente do Instituto de Prev. Social e Desenvolvimento Humano Cuiabá/Ombudsman Social dos Serv. de Cuiabá JOSÉ EUCLIDES DOS SANTOS FILHO CARLOS CARLÃO P. DO NASCIMENTO LUIZ MÁRIO DE BARROS JOSÉ ANTÔNIO ROSA Secretário Municipal de Infra- Secretário Municipal de Educação Auditoria e Controle Interno Diretor Presidente da Agência Estrutura Municipal de Saneamento PREFEITURA MUNICIPAL DE CUIABÁ INSTITUTO DE PLANEJAMENTO E DESENVOLVIMENTO URBANO DIRETORIA DE PESQUISA E INFORMAÇÃO ORGANIZAÇÃO GEOPOLÍTICA DE CUIABÁ • LIMITES MUNICIPAIS • LIMITES DOS DISTRITOS • LIMITE DO PERÍMETRO URBANO • ADMINISTRAÇÕES REGIONAIS • ABAIRRAMENTO Cuiabá, agosto de 2007. 2007. Prefeitura Municipal de Cuiabá/IPDU Ficha catalográfica CUIABÁ. Prefeitura Municipal de Cuiabá / Organização Geopolítica de Cuiabá. -
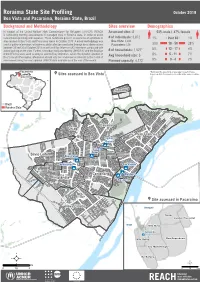
Roraima State Site Profiling. Boa Vista and Pacaraima, Roraima State
Roraima State Site Profiling October 2018 Boa Vista and Pacaraima, Roraima State, Brazil Background and Methodology Sites overview Demographics In support of the United Nations High Commissioner for Refugees (UNHCR), REACH Assessed sites: 8 53% male / 47% female 1+28+4+7+7 is conducting monthly assessments in managed sites in Roraima state, in order to assist 1+30+5+8+9 humanitarian planning and response. These factsheets present an overview of conditions in # of individuals: 3,872 1% 0ver 60 1% sites located in Boa Vista and Pacaraima towns in October 2018. A mixed methodology was Boa Vista: 3,444 used to gather information, with primary data collection conducted through direct observations Pacaraima: 428 30% 18 - 59 28% between 29 and 30 of October 2018 as well as 8 Key Informant (KI) interviews conducted with 5% 12 - 17 4% actors working on the sites. Further, secondary data provided by UNHCR KI and the Brazilian # of households: 1,527* Armed Forces were used to analyse selected key indicators. Given the dynamic situation in Avg household size: 3 8% 5 - 11 7% Boa Vista and Pacaraima, information should only be considered as relevant to the month of assessment using the most updated UNHCR data available as of the end of the month. Planned capacity: 4,172 9% 0 - 4 7% !(Pacaraima *Estimated by assuming an average household size, !( ¥Sites assessed in Boa Vista based on data from previous rounds in the same location. Boa Vista Cauamé Brazil Roraima State Cauamé União São Francisco Jardim Floresta ÔÆ Tancredo Neves Silvio Leite Nova Canaã ÔÆ ÔÆ Pintolândia São Vicente ÔÆ ÔÆ ÔÆ Centenário ÔÆ Rondon 1 Pintolândia Rondon 3 ¥Site assessed in Pacaraima Nova Cidade Venezuela Suapi Jardim Florestal Brazil Vila Nova Janokoida ÔÆ Das Orquídeas Vila Velha Ilzo Montenegro Da Balança ² ² km m 0 1,5 3 0 500 1.000 Fundo de População das Nações Unidas União Europeia Roraima site profiling October 2018 Jardim Floresta Boa Vista, Roraima State, Brazil Lat. -

Evolution of Land Use in the Brazilian Amazon: from Frontier Expansion to Market Chain Dynamics
Land 2014, 3, 981-1014; doi:10.3390/land3030981 OPEN ACCESS land ISSN 2073-445X www.mdpi.com/journal/land/ Article Evolution of Land Use in the Brazilian Amazon: From Frontier Expansion to Market Chain Dynamics Luciana S. Soler 1,2,*, Peter H. Verburg 3 and Diógenes S. Alves 4 1 Land Dynamics Group, Wageningen University, PO Box 47, 6700 AA Wageningen, The Netherlands 2 National Early Warning and Monitoring Centre of Natural Disasters (Cemaden), Parque Tecnológico, Av. Dr. Altino Bondensan 500, São José dos Campos 12247, Brazil 3 Institute for Environmental Studies, VU University Amsterdam, De Boelelaan 1087, Amsterdam 1081 HV, The Netherlands; E-Mail: [email protected] 4 Image Processing Division, National Institute for Space Research (INPE), Av. dos Astronautas 1758, São José dos Campos 12227, Brazil; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +55-12-3186-9236. Received: 31 December 2013; in revised form: 30 July 2014 / Accepted: 7 August 2014 / Published: 19 August 2014 Abstract: Agricultural census data and fieldwork observations are used to analyze changes in land cover/use intensity across Rondônia and Mato Grosso states along the agricultural frontier in the Brazilian Amazon. Results show that the development of land use is strongly related to land distribution structure. While large farms have increased their share of annual and perennial crops, small and medium size farms have strongly contributed to the development of beef and milk market chains in both Rondônia and Mato Grosso. Land use intensification has occurred in the form of increased use of machinery, labor in agriculture and stocking rates of cattle herds. -

Antifouling Adaptations of Marine Shrimp (Decapoda: Caridea): Gill Cleaning Mechanisms and Grooming of Brooded Embryos
Zoological Journal oj the Linnean Society, 6i: 281-305. With 12 figures April 1979 Antifouling adaptations of marine shrimp (Decapoda: Caridea): gill cleaning mechanisms and grooming of brooded embryos RAYMOND T. BAUER Biological Sciences, California Polytechnic State University, San Luis Obispo, California, U.S.A. Accepted for publication September 1977 Gills in the branchial chambers of caridean shrimps, as well as the brooded embryos in females, are subject to fouling by particulate debris and epizoites. Important mechanisms for cleaning the gills are brushing of the gills by the grooming or cleaning chelipeds in some species, while in others, setae from the bases of the thoracic legs brush up among the gills during movement of the limbs (epipod- setobranch complexes). Setae of cleaning chelipeds and of epipod-setobranch complexes show- similar ultrastructural adaptions for scraping gill surfaces. Ablation of the cleaning chelipeds ol the shrimp Heptacarpm pictus results in severe fouling of the gills in experimenials, while those of controls remain clean, Embrvos brooded by female carideans are often brushed and jostled by the grooming chelipeds. In H. pictui. removal of the cleaning chelae results in heavier microbial and sediment fouling than in controls. KEY WO RDS: - shrimp - gills - grooming - cleaning - cpipods - sctobranchs - fouling - Decapoda - Caridea. CONTENTS Introduction 281 Methods 282 Results 284 Gill cleaning by the chelipeds 284 Gill cleaning by the epipod-setobranch complex 289 Experiments on the adaptive value of cheliped brushing in Heptacarpus pictus 292 Cleaning of brooded embryos 296 Experiments on the adaptive value of cheliped brushing of eggs in Heptacarpus pictus 297 Discussion 299 Adaptive value of gill cleaning mechanisms in caridean shrimp 299 Adaptive value of embryo brushing by females 301 Acknowledgements 302 References 302 INTRODUCTION Grooming behaviour is a frequent activity of caridean shrimp which appears to prevent epizoic and sediment fouling of the body (Bauer, 1975; Bauer, 1977, Bauer, 1978). -

Exploratory Temporal and Spatial Distribution Analysis of Dengue Notifications in Boa Vista, Roraima, Brazilian Amazon, 1999-2001†
Exploratory Temporal and Spatial Distribution Analysis of Dengue Notifications in Boa Vista, Roraima, Brazilian Amazon, 1999-2001† by Maria Goreti Rosa-Freitas*, Pantelis Tsouris**#, Alexander Sibajev***, Ellem Tatiani de Souza Weimann**, Alexandre Ubirajara Marques**, Rodrigo Lopes Ferreira** and José Francisco Luitgards-Moura*** *Laboratório de Transmissores de Hematozoários, Departamento de Entomologia, Instituto Oswaldo Cruz, Av. Brasil 4365, Manguinhos, 21045-900 Rio de Janeiro, RJ, Brasil **Núcleo Avançado de Vetores – Convênio FIOCRUZ-UFRR BR 174 S/N - Boa Vista, RR, Brasil ***Centro de Ciências Biológicas e da Saúde, UFRR, BR 174 S/N - Boa Vista, RR, Brasil Abstract In Brazil, as in the rest of the world, dengue has become the most important arthropod-borne viral disease of public health significance. Roraima state presented the highest dengue incidence coefficient in Brazil in the last few years (224.9 and 164.2 per 10,000 inhabitants in 2000 and 2001, respectively). The capital, Boa Vista, reports the highest number of dengue cases in Roraima. This study examined the temporal and spatial distribution of dengue in Boa Vista during the years 1999- 2001, based on daily notifications as recorded by local health authorities. Temporally, dengue notifications were analysed by weekly and monthly averages and age distribution and correlated to meteorological variables. Spatially, dengue coefficients, premises infestation indices, population density and income levels were allocated in a geographical information system using, Boa Vista 49 neighbourhoods as units. Dengue outbreaks displayed distinct year-to-year distribution patterns that were neither periodical nor significantly correlated to any meteorological variable. There were no preferences for age and sex. -

Information Sheet on Ramsar Wetlands (RIS) – 2009-2012 Version Available for Download From
Information Sheet on Ramsar Wetlands (RIS) – 2009-2012 version Available for download from http://www.ramsar.org/ris/key_ris_index.htm. Categories approved by Recommendation 4.7 (1990), as amended by Resolution VIII.13 of the 8th Conference of the Contracting Parties (2002) and Resolutions IX.1 Annex B, IX.6, IX.21 and IX. 22 of the 9th Conference of the Contracting Parties (2005). Notes for compilers: 1. The RIS should be completed in accordance with the attached Explanatory Notes and Guidelines for completing the Information Sheet on Ramsar Wetlands. Compilers are strongly advised to read this guidance before filling in the RIS. 2. Further information and guidance in support of Ramsar site designations are provided in the Strategic Framework and guidelines for the future development of the List of Wetlands of International Importance (Ramsar Wise Use Handbook 14, 3rd edition). A 4th edition of the Handbook is in preparation and will be available in 2009. 3. Once completed, the RIS (and accompanying map(s)) should be submitted to the Ramsar Secretariat. Compilers should provide an electronic (MS Word) copy of the RIS and, where possible, digital copies of all maps. 1. Name and address of the compiler of this form: FOR OFFICE USE ONLY. DD MM YY Beatriz de Aquino Ribeiro - Bióloga - Analista Ambiental / [email protected], (95) Designation date Site Reference Number 99136-0940. Antonio Lisboa - Geógrafo - MSc. Biogeografia - Analista Ambiental / [email protected], (95) 99137-1192. Instituto Chico Mendes de Conservação da Biodiversidade - ICMBio Rua Alfredo Cruz, 283, Centro, Boa Vista -RR. CEP: 69.301-140 2. -

Crustacea, Decapoda, Dendrobranchiata and Caridea) from Off Northeastern Japan
Bull. Natl. Mus. Nat. Sci., Ser. A, 42(1), pp. 23–48, February 22, 2016 Additional Records of Deep-water Shrimps (Crustacea, Decapoda, Dendrobranchiata and Caridea) from off Northeastern Japan Tomoyuki Komai1 and Hironori Komatsu2 1 Natural History Museum and Institute, Chiba, 955–2 Aoba-cho, Chiba 260–8682, Japan E-mail: [email protected] 2 Department of Zoology, National Museum of Nature and Science, 4–1–1 Amakubo, Tsukuba, Ibaraki 305–0005, Japan E-mail: [email protected] (Received 5 October 2015; accepted 22 December 2015) Abstract Four deep-water species of shrimps are newly recorded from off Tohoku District, northeastern Japan: Hepomadus gracialis Spence Bate, 1888 (Dendrobranchiata, Aristeidae), Pasiphaea exilimanus Komai, Lin and Chan, 2012 (Caridea, Pasiphaeidae), Nematocarcinus longirostris Spence Bate, 1888 (Caridea, Nematocarcinidae), and Glyphocrangon caecescens Anonymous, 1891 (Caridea, Glyphocrangonidae). Of them, the bathypelagic P. exilimanus is new to the Japanese fauna. The other three species are abyssobenthic, extending to depths greater than 3000 m, and thus have been rarely collected. The newly collected samples of H. gracialis enable us to reassess diagnostic characters of the species. Nematocarcinus longirostris is rediscovered since the original description, and the taxonomy of the species is reviewed. Glyphocrangon caecescens is the sole representative of the family extending to off northern Japan. Key words : Hepomadus gracialis, Pasiphaea exilimanus, Nematocarcinus longirostris, Glypho- crangon caecescens,