Masterton Wastewater Treatment Plant Health Impact Assessment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
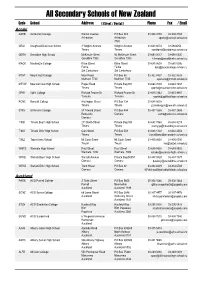
Secondary Schools of New Zealand
All Secondary Schools of New Zealand Code School Address ( Street / Postal ) Phone Fax / Email Aoraki ASHB Ashburton College Walnut Avenue PO Box 204 03-308 4193 03-308 2104 Ashburton Ashburton [email protected] 7740 CRAI Craighead Diocesan School 3 Wrights Avenue Wrights Avenue 03-688 6074 03 6842250 Timaru Timaru [email protected] GERA Geraldine High School McKenzie Street 93 McKenzie Street 03-693 0017 03-693 0020 Geraldine 7930 Geraldine 7930 [email protected] MACK Mackenzie College Kirke Street Kirke Street 03-685 8603 03 685 8296 Fairlie Fairlie [email protected] Sth Canterbury Sth Canterbury MTHT Mount Hutt College Main Road PO Box 58 03-302 8437 03-302 8328 Methven 7730 Methven 7745 [email protected] MTVW Mountainview High School Pages Road Private Bag 907 03-684 7039 03-684 7037 Timaru Timaru [email protected] OPHI Opihi College Richard Pearse Dr Richard Pearse Dr 03-615 7442 03-615 9987 Temuka Temuka [email protected] RONC Roncalli College Wellington Street PO Box 138 03-688 6003 Timaru Timaru [email protected] STKV St Kevin's College 57 Taward Street PO Box 444 03-437 1665 03-437 2469 Redcastle Oamaru [email protected] Oamaru TIMB Timaru Boys' High School 211 North Street Private Bag 903 03-687 7560 03-688 8219 Timaru Timaru [email protected] TIMG Timaru Girls' High School Cain Street PO Box 558 03-688 1122 03-688 4254 Timaru Timaru [email protected] TWIZ Twizel Area School Mt Cook Street Mt Cook Street -

Education Region (Total Allocation) Cluster Name School Name School
Additional Contribution to Base LSC FTTE Whole Remaining FTTE Total LSC for Education Region Resource (Travel Cluster Name School Name School Roll cluster FTTE based generated by FTTE by to be allocated the Cluster (A (Total Allocation) Time/Rural etc) on school roll cluster (A) school across cluster + B) (B) Coley Street School 227 0.45 Foxton Beach School 182 0.36 Foxton School 67 0.13 Kerekere Community of Learning 2 2 0 2 Manawatu College 309 0.62 Shannon School 73 0.15 St Mary's School (Foxton) 33 0.07 Chanel College 198 0.40 Douglas Park School 329 0.66 Fernridge School 189 0.38 Hadlow Preparatory School 186 0.37 Lakeview School 382 0.76 Makoura College 293 0.59 Masterton Intermediate 545 1.09 1 Mauriceville School 33 0.07 Opaki School 193 0.39 Masterton (Whakaoriori) Kāhui Ako 10 7 0 10 Rathkeale College 317 0.63 Solway College 154 0.31 Solway School 213 0.43 St Matthew's Collegiate (Masterton) 311 0.62 St Patrick's School (Masterton) 233 0.47 Tinui School 33 0.07 Wainuioru School 82 0.16 Wellington Wairarapa College 1,080 2.16 2 Whareama School 50 0.10 Avalon School 221 0.44 Belmont School (Lower Hutt) 366 0.73 Dyer Street School 176 0.35 Epuni School 93 0.19 Kimi Ora School 71 0.14 Naenae Community of Learning 5 4 0 5 Naenae College 705 1.41 1 Naenae Intermediate 336 0.67 Naenae School 249 0.50 Rata Street School 348 0.70 St Bernadette's School (Naenae) 113 0.23 Bellevue School (Newlands) 308 0.62 Newlands College 1,000 2.00 2 Newlands Intermediate 511 1.02 1 Newlands Community of Learning 5 2 0 5 Newlands School 310 0.62 Paparangi -

14/03/2019 Newtown Park Stadium, Wellington Results Event 114 Boys
Newtown Park, Wellington - Site License Hy-Tek's MEET MANAGER 9:12 AM 15/03/2019 Page 1 CSW Regional Championships 2019 - 14/03/2019 Newtown Park Stadium, Wellington Results Event 114 Boys 100 Meter Run Junior Record: 11.73 R 23/03/2017 Joshua Williams, Wgtn Coll Name Team Prelims Wind H# Preliminaries 1 Lambert, Ben Paraparaumu College 11.83 q 2.4 1 2 Tofts, Max Paraparaumu College 12.29 q 0.7 2 3 Skelton, Sebastian Scots College 12.31 q 2.4 1 4 Tyrell, Mark St Patrick's College Town 12.45 q 0.7 2 5 Broderick, Lennox Wairarapa College 12.46 q 2.4 1 6 Su'a, Jack St Patrick's College Town 12.50 q 0.7 2 7 Wolff, Oskar Kapiti College 12.55 q 2.4 1 8 Clark, Alexander Wairarapa College 12.56 q 0.7 2 9 Sheehan, Anthony Wellington College 12.63 q 2.4 1 10 Wakeling, Zane Kapiti College 12.65 0.7 2 11 Solomon, Stanley Wellington College 12.67 0.7 2 12 Perez, Aleki St Patrick's Silverstream 12.74 2.4 1 13 Debriacher, Andre St Bernard's College 13.09 0.7 2 14 Hunt, Phoenix Heretaunga College 13.31 2.4 1 15 Hunapo-Harrison, Tama Heretaunga College 13.40 2.4 1 16 Urwin, Eli Heretaunga College 13.53 0.7 2 Event 114 Boys 100 Meter Run Junior Record: 11.73 R 23/03/2017 Joshua Williams, Wgtn Coll Name Team Finals Wind Finals 1 Lambert, Ben Paraparaumu College 11.86 -0.5 2 Tyrell, Mark St Patrick's College Town 12.28 -0.5 3 Tofts, Max Paraparaumu College 12.37 -0.5 4 Broderick, Lennox Wairarapa College 12.46 -0.5 5 Skelton, Sebastian Scots College 12.57 -0.5 6 Clark, Alexander Wairarapa College 12.57 -0.5 7 Su'a, Jack St Patrick's College Town 12.61 -0.5 -

Opaki 1868 1873 1876
Opaki Plain, 30 ; Castle Point and Alfredton, 80. Total, 500. The Mercury says :— Calling out the Militia has strengthened the number of Opaki: 1878-Present. Opaki School was originally further North volunteers very materially, and many men who object to serve in the by Mikimiki Road and when Mikimiki School was opened the Militia hasten to enrol themselves (entirely regardless of the school was moved to be near the Railway Station. I can find no meaning of the word) as Volunteers. The Greytown Infantry has mention of an Upper Opaki School as I have seen written. A number increased the number of its men, owing to a threat that if the of references to this are to do with the Original Opaki School and company did not comprise a sufficient number, the members would then Mikimiki. 6 kilometres north of Masterton just off State be enrolled as Militiamen; while the members of the Cavalry found Highway 2 they could be independent of Captain Donald's troop, and form one of their own. A similar increase has been noticed in the other Volunteer Companies, and we must agree with a remark made by the Adjutant, that there is no recruiting sergeant for the Volunteers so efficient as the Militia being in the back ground PP 1873 3rd June 1873 In another week's time that great boon to Masterton, the Waipoua Bridge, will be completed, which throws open the Opaki Block, and pedestrians will be able to travel and enjoy the scenery, and visit the various rising farms, where more corn is grown than in in any other part of the Wairarapa- The frontages to the main road near the bridge have already risen in value. -
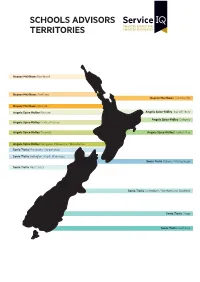
Schools Advisors Territories
SCHOOLS ADVISORS TERRITORIES Gaynor Matthews Northland Gaynor Matthews Auckland Gaynor Matthews Coromandel Gaynor Matthews Waikato Angela Spice-Ridley Waikato Angela Spice-Ridley Bay of Plenty Angela Spice-Ridley Gisborne Angela Spice-Ridley Central Plateau Angela Spice-Ridley Taranaki Angela Spice-Ridley Hawke’s Bay Angela Spice-Ridley Wanganui, Manawatu, Horowhenua Sonia Tiatia Manawatu, Horowhenua Sonia Tiatia Welington, Kapiti, Wairarapa Sonia Tiatia Nelson / Marlborough Sonia Tiatia West Coast Sonia Tiatia Canterbury / Northern and Southern Sonia Tiatia Otago Sonia Tiatia Southland SCHOOLS ADVISORS TERRITORIES Gaynor Matthews NORTHLAND REGION AUCKLAND REGION AUCKLAND REGION CONTINUED Bay of Islands College Albany Senior High School St Mary’s College Bream Bay College Alfriston College St Pauls College Broadwood Area School Aorere College St Peters College Dargaville High School Auckland Girls’ Grammar Takapuna College Excellere College Auckland Seven Day Adventist Tamaki College Huanui College Avondale College Tangaroa College Kaitaia College Baradene College TKKM o Hoani Waititi Kamo High School Birkenhead College Tuakau College Kerikeri High School Botany Downs Secondary School Waiheke High School Mahurangi College Dilworth School Waitakere College Northland College Diocesan School for Girls Waiuku College Okaihau College Edgewater College Wentworth College Opononi Area School Epsom Girls’ Grammar Wesley College Otamatea High School Glendowie College Western Springs College Pompallier College Glenfield College Westlake Boys’ High -

Friday 18 October 2019 Duration: 9.03Am-3.28Pm Morning Tea: 10.35Am-10.57Am Lunch: 12.35Pm-1.30Pm
New Zealand Geographic Board Ngā Pou Taunaha o Aotearoa (NZGB) MINUTES Venue: Pōhutukawa and Nikau meeting rooms Level 7, Radio New Zealand House 155 The Terrace Wellington Friday 18 October 2019 Duration: 9.03am-3.28pm Morning tea: 10.35am-10.57am Lunch: 12.35pm-1.30pm NOTE: All information recorded in these Minutes relating to Treaty of Waitangi settlement place name proposals is confidential and is not available to the general public. Some of the information may become available after Deeds of Settlement are signed. General 1. Karakia | Welcome Matanuku Mahuika opened the hui with a karakia. Obituaries Matanuku Mahuika acknowledged the recent passing of Lee Smith of Ngāti Kahungunu and noted the assistance as a te reo Māori translator that Lee Smith had given to the NZGB at various times. The Chairperson acknowledged the recent passing of Tahu Potiki, former Chief Executive of Te Rūnanga o Ngāi Tahu. Welcome The Chairperson welcomed everyone to the hui. He welcomed observer Philip Green from Te Arawhiti and advised that other representatives from Te Arawhiti would join the meeting when agenda item 11 ‘Treaty advice’ and item 9 ‘Standard for Crown Protected Area names’ are discussed. He also welcomed Daniel Wainwright, on secondment as the NZGB’s Advisor responsible for Treaty Names, and other members of the NZGB Secretariat. The Chairperson congratulated Adrienne Staples on her reappointment to Wellington Regional Council. The Chairperson noted that Paulette Tamati-Elliffe would be late arriving due to flight disruption. 2. Present | Apologies NZGB members (9) Anselm Haanen, Chairperson Surveyor-General, Land Information New Zealand (LINZ) Adam Greenland National Hydrographer, LINZ David Barnes Federated Mountain Clubs of New Zealand Inc. -

Penton Mangapakeha 1895
Writing in blue is notes from School Committee minute books 1st March 1898 Plans for Mangapakeha School may also "be seen at Writing in Tempus Sans ITC is material researched at National the residence of Mr. McLeay Penton, Mangapakeha PP rd Archives 23 February 1898 The Chairman was authorised to open tenders for schools at (Te) Wharau, Te Ore Ore, and Penton, to accept the lowest tender PP 8th May 1901 Teacher is required for the Aided School at Penton, near Masterton. Salary £3O, and residence Applications to be sent to Mr. J. McLeay, Penton via Masterton PP (It is clear that Penton School and Mangapakeha School are interchangeable terms) Mangapakeha: 1898-1962 This school always struggled for pupils. When the building was first erected the locals continued their school elsewhere. There were problems with teachers appointed by locals. Situated 34 Kilometres from Masterton. The building is used at the Cobblestones Museum in Greytown Note: Google Maps has Mangapakeha on the Masterton side of the turnoff to Riversdale closer to the Rewanui Forest Park, but the school was definitely much closer to Tinui about where marked Mangapakeha Penton 1895- 1900 th 30 June 1897 The Inspector was instructed to report as to the 15th October An aided school is being established at the Mangapakeha requirement for rebuilding the aided School at Penton, which had junction been burnt down. 30 June 1897 PP 30th January 1895 Mr Donald Sinclair was appointed to the charge of the 26th January 1898 It was decided to establish an aided school at Mangapakeha aided school Penton, in the Mangapakeha district, where there were 18 children of 31st January The aided school at Mangapakeha is to be opened next school age and seven under school age PP Monday. -

AGENDA Audit and Risk 2019-08-21 Website
MASTERTON DISTRICT COUNCIL AGENDA AUDIT AND RISK COMMITTEE WEDNESDAY 21 AUGUST 2019 AT 3.00PM MEMBERSHIP OF THE COMMITTEE Philip Jones (Chairperson) Her Worship the Mayor Cr D Davidson Cr J Dalziell Cr B Goodwin Cr G McClymont Cr C Peterson Quorum: Four Noce is given that the ̀ meeng of the Masterton District Council Audit and Risk Commi. ee will be held on Wednesday 21 August 2019 at REAP House, 340 Queen St, Masterton commencing at 3.00pm. RECOMMENDATIONS IN REPORTS ARE NOT TO BE CONSTRUED AS COUNCIL POLICY UNTIL ADOPTED 18 August 2019 1 AGENDA ITEMS 1. APOLOGIES 2. CONFLICTS OF INTEREST (Members to declare conflicts, if any) 3. LATE ITEMS 4. MINUTES OF PREVIOUS MEETING TO BE RECEIVED (092/19) Pages 401-406 FOR INFORMATION 5. FOURTH QUARTER REPORTS (166/19) Pages 411-418 a) Destination Wairarapa - 12 month report and financial statements Pages 419-436 b) Connecting Communities - six month report and financial statements Pages 437-448 c) Sport Wellington Wairarapa - Annual Report Pages 449-463 d) Aratoi Regional Trust - quarterly report and financial statements Pages 464-470 6. NON-FINANCIAL PERFORMANCE 2017/2018 QUARTER 4 (167/19) Pages 471-486 7. ANNUAL REPORT, AUDIT PLAN & TIMETABLE (168/19) Pages 487-501 8. RATES RECEIVABLE REPORT (169/19) Pages 502-504 9. TREASURY MANAGEMENT REPORT (170/19) Pages 505-510 10. BUSINESS IMPROVEMENT WORK PROGRAMME (171/19) Pages 511-517 11. PRIVACY POLICY AND ENFORCEMENT POLICY (172/19) Pages 518-544 12 HEALTH & SAFETY QUARTERLY REPORT (173/19) Pages 545-551 13. NZTA INVESTMENT AUDIT REPORT (174/19) Pages 552-571 14. -

2015 Wairarapa Secondary Schools T&F Champs
2015 Wairarapa Secondary Schools T&F Champs - 11/03/2015 Pelorus Trust Athletics Track, Colin Pugh Sports Bowl, Masterton, Intermediate Girls Hammer ================================================================== ======== Name Yr Team Mark Wind Pts -------------------------------------------------------------------------- 1 Berenique Erasmus Chanel College 20.73m NWI -- 2 Grace Hymers Chanel College 9.91m NWI -- NT Holly Bird Wairarapa College ND Senior Girls Hammer ================================================================== ======== Name Yr Team Mark Wind Pts -------------------------------------------------------------------------- 1 Grace Quinn Solway College 41.06m NWI -- 2 Totisa Tomane Solway College 23.08m NWI -- NT Emma Kruszona Wairarapa College ND NT Severa Masoe Chanel College ND Intermediate Boys Hammer ================================================================== ======== Name Yr Team Mark Wind Pts -------------------------------------------------------------------------- 1 Nathan Whiteman Rathkeale College 33.79m NWI -- 2 Cameron Clatworthy Rathkeale College 24.56m NWI -- Senior Boys Hammer ================================================================== ======== Name Yr Team Mark Wind Pts -------------------------------------------------------------------------- 1 Vincent Capes Rathkeale College 25.68m NWI -- NT Jordan Ellicock Wairarapa College ND NT Sam Siaosi Chanel College ND Junior Girls Long Jump ================================================================== =========== Name Yr Team Mark Wind -

Pitopito Kōrero
Wairarapa Wearable Arts 2013 Pitopito kōrero Quarterly newsletter from Public Health (Wairarapa) August 2013 Issue 4 Public Health: working with you in our community The Wairarapa Wearable Arts awards evening was held at The smokefree category was one of seven, the others Makoura College, Thursday 22 August and attracted 100 categories were: Intermediate Open - for younger entries from all over the Wairarapa. Contributing schools students; Upcycled - for those wishing to recycle clothing or included Chanel College, Kuranui College, Lakeview other materials; Masks - in which students made statements School, Makoura College, Masterton Intermediate School, with their face coverings; Turangawaewae - which allowed Solway College and Wairarapa College. students to investigate their own identity; Time Travel - which gave students the chance to look at old and new This year the Wairarapa Wearable Arts included a new The Wairarapa Public Health Unit (closely linked to fashions and the way time passes and finally a UV section - category Smoke Free vision 2025. This category was Regional Public Health) works to keep the community which let students glow in the dark. Wairarapa Blokes’ launch sponsored by the Smokefree Network of Wairarapa healthy by providing services that promote good health to promote its vision that future generations of New A big well done to the Smokefree category practices and prevent disease. Zealand children will be free from exposure to tobacco prize-winners!!! We go where you work, learn, live and play to help you and will enjoy smokefree lives (by 2025), and to bring stay healthy; health begins before you need medical care. SMOKE FFREE VISION 2025 creative flare to NZ’s dying pastime (no pun intended). -
DRAFT LANE DRAW Waka Ama - Hoe Tonga Secondary School Sprint Champs Sat, 13 Mar 2021
DRAFT LANE DRAW Waka Ama - Hoe Tonga Secondary School Sprint Champs Sat, 13 Mar 2021 W1 races over 250m 001 Girls U16 - W1 250m Heat 1 / 3 9:00 AM 1 Te Kura o iwi Whakatupuranga Rua Mano SUITAULOA, Trilaine 2 Solway College SOMERVILLE, Xanthe 3 Mana College MATAPUKU-KITISENI, L A 4 St Mary's College SCHWALGER, Phoenix 002 Girls U16 - W1 250m Heat 2 / 3 9:06 AM 1 Te Kura Maori o Porirua MATAKATEA, Te Ohomairangi 2 Mana College RUWHIU, Tiana 3 Wellington High School BEAGLEHOLE, Vidthia 4 003 Girls U16 - W1 250m Heat 3 / 3 9:12 AM 1 Te Kura Maori o Porirua PAAMA-AKAVI, Keana 2 Sacred Heart College (Wgtn) BARBER, Nareiah 3 Manukura DONALDSON, Piper 4 Manukura TATANA-WAIHI, Koanga 004 Boys U16 - W1 250m Heat 1 / 3 9:18 AM 1 Rathkeale College MACKAY, Kaahurere 2 Manukura ELLIOT-TOMAN, Wairehu 3 Manukura RAKURAKU, Raumati 4 005 Boys U16 - W1 250m Heat 2 / 3 9:24 AM 1 Rongotai College CARTER, Mahe 2 Manukura HEREWINI, Deilorenze 3 Te Kura o iwi Whakatupuranga Rua Mano HENARE, Manaia-Dupree 4 006 Boys U16 - W1 250m Heat 3 / 3 9:30 AM 1 Te Kura Maori o Porirua THOMAS, Nikau 2 Te Kura Maori o Porirua PAAMA-AKAVI, Jean-Marie 3 Bishop Viard College SCHWAGLER, Z'Kdeus 4 007 Girls U19 - W1 250m Heat 1 / 2 9:36 AM 1 Te Kura o iwi Whakatupuranga Rua Mano HENARE, Te Rangihuia 2 Sacred Heart College (Wgtn) BARBER, Maia-Sian 3 Wellington East Girls' College SO'O, Le'aniva 4 Mana College MUCALO-WAIARIKI, Kohine EnterNOW RaceManager Licenced to: Hoe Tonga 1 of 8 Printed: Mon, 08 Mar 10:20:07 PM DRAFT LANE DRAW Waka Ama - Hoe Tonga Secondary School Sprint Champs -
Council Agenda
MASTERTON DISTRICT COUNCIL COUNCIL AGENDA MEMBERSHIP Her Worship (Chairperson) Cr G Caffell Cr B Johnson Cr J Dalziell Cr G McClymont Cr D Davidson Cr F Mailman Cr B Goodwin Cr S O’Donoghue Cr J Hooker Cr C Peterson Noce is given that a meeng of the Masterton District Council will be held at REAP House, 340 Queen St, Masterton on Wednesday 7 August 2019 at 2.00pm. RECOMMENDATIONS IN REPORTS ARE NOT TO BE CONSTRUED AS COUNCIL POLICY UNTIL ADOPTED 2 August 2019 Values 1. Public interest: members will serve the best interests of the people within the Masterton district and discharge their duties conscientiously, to the best of their ability. 2. Public trust: members, in order to foster community confidence and trust in their Council, will work together constructively and uphold the values of honesty, integrity, accountability and transparency. 3. Ethical behaviour: members will not place themselves in situations where their honesty and integrity may be questioned, will not behave improperly and will avoid the appearance of any such behaviour. 4. Objectivity: members will make decisions on merit; including appointments, awarding contracts, and recommending individuals for rewards or benefits. 5. Respect for others: will treat people, including other members, with respect and courtesy, regardless of their ethnicity, age, religion, gender, sexual orientation, or disability. Members will respect the impartiality and integrity of Council staff. 6. Duty to uphold the law: members will comply with all legislative requirements applying to their role, abide by this Code, and act in accordance with the trust placed in them by the public.