Microsurgery: Free Tissue Transfer and Replantation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intestinal Staple Line Reinforcement Using Matristem
Surgical Science, 2015, 6, 65-70 Published Online February 2015 in SciRes. http://www.scirp.org/journal/ss http://dx.doi.org/10.4236/ss.2015.62011 Intestinal Staple Line Reinforcement Using MatriStem Kent C. Sasse, David Warner*, Sean M. Ward, Walter Mandeville, Rebecca Evans Department of Physiology & Cell Biology, University of Nevada School of Medicine, Reno, USA Email: *[email protected] Received 31 December 2014; accepted 10 February 2015; published 13 February 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Background: Staple line reinforcement material has been demonstrated to raise the burst pres- sure threshold after linear intestinal stapling. Numerous bioprosthetic materials have been uti- lized in surgical practice. Porcine urinary bladder matrix (ACell, Inc.) is an extracellular matrix material derived from porcine bladder used to reinforce surgically repaired soft tissue, and facili- tate the body’s regenerative capacity. Objective: This study represents the first evaluation of uri- nary bladder matrix in gastrointestinal staple line reinforcement. Methods: Pathogen-free pigs underwent midline laparotomy under general anesthesia. Small intestinal division was performed with an endoscopic linear stapler. Nineteen intestinal divisions were performed with urinary bladder matrix staple line reinforcement, and twenty divisions were unreinforced. Staple lines were then subjected to burst pressure analysis by intraluminal infusion of dyed Krebs solution at an infusion rate of 20 ml·min−1 under manometric monitoring. Upon visible staple line extravasa- tion, intraluminal pressure was recorded. Results: Intestinal staple lines reinforced with urinary bladder matrix exhibited significantly higher burst pressure threshold (p < 0.05). -

Urotoday International Journal®
UIJ UroToday International Journal® Percutaneous Dilatation of Non-malignant Ureteroenteric Anastomotic Strictures in Patients with Urinary Diversion After Cystectomy for Bladder Cancer: 7 Patients Sallami Satáa, Olfa Zrayer, Habiba Mizouni, Sami Ben Rhouma, Mohamed Hmidi, Mourad Gargaouri, Maher Chtourou Submitted June 18, 2012 - Accepted for Publication July 23, 2012 ABSTRACT Background: The management of ureterointestinal stricture in patients who have undergone urinary diversion can be challenging. Endourological techniques have been increasingly used in recent years for such strictures. Objectives: We report our experience and evaluate our results on balloon antegrade dilatations for benign ureteroenteric anastomotic strictures after total cystectomy and urinary diversion by ileal conduit. Patients and Methods: Between December 1990 and May 2009, 8 balloon dilatations were performed on 7 patients with a mean age of 56.6 years (range: 50 to 72) to treat ureterointestinal strictures. Strictures were dilated percutaneously via the antegrade approach under fluoroscopic control. A ureteral multi-hole catheter was left for 6 to 8 weeks. Success was defined as radiological resolution of obstruction and the ability to recover normal activity in the absence of flank pain, infection, or the need for ureteral stents or nephrostomy tubes. Results: The development of strictures occurred a mean of 4.5 months after urinary diversion. Eight renal units were treated (5 left, 3 right), including 1 bilateral procedure. There were 6 complete and 2 partial strictures. The operative time did not exceed 45 minutes. No major complications were encountered during or after these procedures. The overall success rate was 43%. Three patients required open reimplantation. Six of 7 patients showed satisfactory outcomes and 1 patient was lost to follow-up. -

The Use of Cyanoacrylate in Surgical Anastomosis: an Alternative to Microsurgery
[Downloaded free from http://www.jstcr.org on Tuesday, June 29, 2010, IP: 41.185.132.78] SUrGiCAL opiNioN The Use of Cyanoacrylate in Surgical Anastomosis: An Alternative to Microsurgery G. M. Bot, K. G. Bot1, J. O. Ogunranti2, J. A. Onah2, A. Z. Sule, I. Hassan3, E. D. Dung Department of Surgery, Jos University Teaching Hospital, ABSTRACT 1Faculty of Medical Science, University of Jos, 2Department of Anatomy, University of Jos, 3Department of Surgery, To present anastomosis with cyanoacrylate as a cheap, Aminu Kano University Teaching Hospital, Plateau State, simple, fast, and available technique for anastomosis in Nigeria urological, vascular, gynecological, and general surgical procedures. This method may in the future be a good alternative to microsurgery, particularly in centers where INTRODUCTION facilities are unavailable and the financial implication is unbearable for the patient. Cyanoacrylate is an adhesive or glue that is available in different chemical forms ranging from he use of cyanoacrylate is not widespread despite ethylcyanoacrylate (superglue) to Isobutylcyanoacrylate a number of published studies on the subject.[1] and octylcyanoacrylate (dermerbond), which is in TCyanoacrylate is an adhesive that was first clinical use. Anastomosis with cyanoacrylate requires discovered by Dr. Harry Coover and Fred Joyner in the the application of stay sutures, a luminal stent and the [2] subsequent application of the adhesive. The adhesives with Kodak laboratory. Cyanoacrylates were first synthesized lower molecular weights produce a rigid and patent region by Airdis in 1949, while Coover et al. described their of anastomosis, while the higher molecular compounds adhesive property and suggested their possible use as produce a consistency close to the normal tissue. -

The Plastic Surgery Research Council 59Th Annual Meeting Abstract Supplement EDITORIAL BOARD
PSRC Supplement Abstracts Online at www.PRSJournal.comSee the VOLUME 133 • NUMBER 3S • MARCH 2014 SUPPLEMENT www.PRSJournal.com Supplement to Plastic and Reconstructive Surgery ® Journal of the American Society of Plastic Surgeons Official Organ of the American Association of Plastic Surgeons Official Organ of the American Society of Maxillofacial Surgeons Official Organ of the Plastic Surgery Research Council The Plastic Surgery Research Council 59th Annual Meeting Abstract Supplement EDITORIAL BOARD Plastic and Reconstructive Surgery EDITOR-IN-CHIEF ASSOCIATE EDITORS SECTION EDITORS Rod J. Rohrich, M.D. William Peter Adams, Jr ., Department of Plastic Surgery Maurice Nahabedian University of Texas Dallas, Texas Washington, D.C. BREAST Southwestern Medical Center Al Aly, Orange, Calif. 5959 Harry Hines Blvd., POB1 David A. Hidalgo Suite 300 Charles E. Butler, Houston, Texas COSMETIC Dallas, Texas 75390-8820 New York, N.Y. [email protected] Grant W. Carlson, Atlanta, Ga. David W. Chang, Chicago, Ill. Paul S. Cederna CO-EDITOR EXPERIMENTAL James M. Stuzin, M.D. Peter G. Cordeiro, New York, N.Y. Ann Arbor, Mich. 3225 Aviation Avenue, Suite 100 Coconut Grove, Fla. 33133-4753 Joseph J. Disa, New York, N.Y. Matthew J. Concannon HAND/ REVIEW EDITOR Gregory A. Dumanian, Chicago, Ill. Columbia, Mo. PERIPHERAL NERVE Ronald P. Gruber, M.D. Steven Fagien, Boca Raton, Fla. 3318 Elm Street (Oculoplastic Surgeon) Scott P. Bartlett PEDIATRIC/ Oakland, Calif. 94609-3012 Philadelphia, Pa. Jeffrey A. Fearon, Dallas, Texas CRANIOFACIAL ASPS ADVERTISING EDITOR Robert D. Galiano, Chicago, Ill. Dennis P. Orgill Charles N. Verheyden, M.D., Bahman Guyuron, Lyndhurst, Ohio Boston, Mass. RECONSTRUCTIVE Ph.D. 2401 S. -
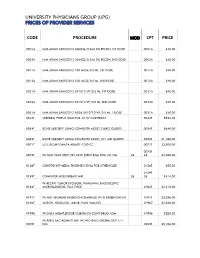
Code Procedure Cpt Price University Physicians Group
UNIVERSITY PHYSICIANS GROUP (UPG) PRICES OF PROVIDER SERVICES CODE PROCEDURE MOD CPT PRICE 0001A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 1ST DOSE 0001A $40.00 0002A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 2ND DOSE 0002A $40.00 0011A IMM ADMN SARSCOV2 100 MCG/0.5 ML 1ST DOSE 0011A $40.00 0012A IMM ADMN SARSCOV2 100 MCG/0.5 ML 2ND DOSE 0012A $40.00 0021A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 1ST DOSE 0021A $40.00 0022A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 2ND DOSE 0022A $40.00 0031A IMM ADMN SARSCOV2 AD26 5X10^10 VP/0.5 ML 1 DOSE 0031A $40.00 0042T CEREBRAL PERFUS ANALYSIS, CT W/CONTRAST 0042T $954.00 0054T BONE SURGERY USING COMPUTER ASSIST, FLURO GUIDED 0054T $640.00 0055T BONE SURGERY USING COMPUTER ASSIST, CT/ MRI GUIDED 0055T $1,188.00 0071T U/S LEIOMYOMATA ABLATE <200 CC 0071T $2,500.00 0075T 0075T PR TCAT PLMT XTRC VRT CRTD STENT RS&I PRQ 1ST VSL 26 26 $2,208.00 0126T CAROTID INT-MEDIA THICKNESS EVAL FOR ATHERSCLER 0126T $55.00 0159T 0159T COMPUTER AIDED BREAST MRI 26 26 $314.00 PR RECTAL TUMOR EXCISION, TRANSANAL ENDOSCOPIC 0184T MICROSURGICAL, FULL THICK 0184T $2,315.00 0191T PR ANT SEGMENT INSERTION DRAINAGE W/O RESERVOIR INT 0191T $2,396.00 01967 ANESTH, NEURAXIAL LABOR, PLAN VAG DEL 01967 $2,500.00 01996 PR DAILY MGMT,EPIDUR/SUBARACH CONT DRUG ADM 01996 $285.00 PR PERQ SAC AGMNTJ UNI W/WO BALO/MCHNL DEV 1/> 0200T NDL 0200T $5,106.00 PR PERQ SAC AGMNTJ BI W/WO BALO/MCHNL DEV 2/> 0201T NDLS 0201T $9,446.00 PR INJECT PLATELET RICH PLASMA W/IMG 0232T HARVEST/PREPARATOIN 0232T $1,509.00 0234T PR TRANSLUMINAL PERIPHERAL ATHERECTOMY, RENAL -

Psi Technical Specs V31.Pdf
AHRQ Quality Indicators Patient Safety Indicators: Technical Specifications Department of Health and Human Services Agency for Healthcare Research and Quality http://www.qualityindicators.ahrq.gov March 2003 Version 3.1 (March 12, 2007) AHRQ Quality Indicators Web Site: http://www.qualityindicators.ahrq.gov Table of Contents About the Patient Safety Indicators ............................................................................................................... 1 Patient Safety Indicators – Detailed Definitions ............................................................................................ 3 Complications of Anesthesia (PSI 1) ............................................................................................................ 3 Death in Low-Mortality DRGs (PSI 2) ........................................................................................................... 5 Decubitus Ulcer (PSI 3) ................................................................................................................................. 7 Failure to Rescue (PSI 4) .............................................................................................................................. 9 Foreign Body Left during Procedure, Secondary Diagnosis Field (PSI 5 and 21)...................................... 17 Iatrogenic Pneumothorax, Secondary Diagnosis Field (PSI 6 and 22)....................................................... 18 Selected Infections Due to Medical Care, Secondary Diagnosis Field (PSI 7 and 23) ............................. -

Robotics in Neurosurgery
The American Journal of Surgery 188 (Suppl to October 2004) 68S–75S Robotics in neurosurgery Paul B. McBeth, M.A.Sc., Deon F. Louw, M.D., Peter R. Rizun, B.A.Sc., Garnette R. Sutherland, M.D.* The Seaman Family MR Research Center, Division of Neurosurgery, Department of Clinical Neurosciences, University of Calgary, 1403 29th Street N.W., Calgary, Alberta T2N 2T9, Canada Abstract Technological developments in imaging guidance, intraoperative imaging, and microscopy have pushed neurosurgeons to the limits of their dexterity and stamina. The introduction of robotically assisted surgery has provided surgeons with improved ergonomics and enhanced visualization, dexterity, and haptic capabilities. This article provides a historical perspective on neurosurgical robots, including image- guided stereotactic and microsurgery systems. The future of robot-assisted neurosurgery, including the use of surgical simulation tools and methods to evaluate surgeon performance, is discussed. Heavier-than-air flying machines are impossible. for holding and manipulating biopsy cannulae. Although the —Lord Kelvin (William Thomson), robot served only as a holder/guide, the potential value of Australian Institute of Physics, 1895 robotic systems in surgery was evident. In 1991, Drake and coworkers [3] reported the use of a PUMA robot as a Neurosurgeons, constrained by their anthropomorphic de- retraction device in the surgical management of thalamic sign, may have reached the limits of their dexterity and astrocytomas. Despite their novel application, both systems stamina. The combination of magnification of the operative lacked the proper safety features needed for widespread field and tool miniaturization has overwhelmed the spatial acceptance into neurosurgery. Beginning in 1987, Benabid resolution of the adult human hand. -

A Fo Dh Ww Doh L
THE EFFECTS OF INDWELLING TRANSURETHRAL CATHETERIZATION AND TUBE CYSTOSTOMY ON URETHRAL ANASTOMOSES IN DOGS by Anjyilla Joye Cooley Thesis submitted to the Faculty of the Virginia Polytechnical Institute and State University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Veterinary Medical Sciences APPROVED: — 7). W——_. Don R. Waldron, Chairman Mark M. Smith refory C. Troy a fo Dh Ww Doh L. Barber May, 1996 Blacksburg, Virginia KEY WORDS: Urethra, wound healing, urinary diversion Mad es panel THE EFFECTS OF INDWELLING TRANSURETHRAL CATHETERIZATION AND TUBE CYSTOSTOMY ON URETHRAL ANASTOMOSES IN DOGS by Anjilla Joye Cooley Committee Chairman: Don R. Waldron Veterinary Medical Sciences This study compared the effects of urinary diversion by tube cystostomy catheterization, urethral catheterization and tube cystostomy and urethral catheterization on healing urethral anastomoses in the canine urethra. Fifteen intact, mature males were divided into three groups of five dogs. Urodynamic studies were performed under halothane anesthesia preoperatively and at ten weeks postoperatively. Urethral anastomosis was performed in all dogs over a urethral catheter with 4-0 polyglyconate. Group U dogs (n=5) received transurethral catheters. Group C dogs (n=5) received tube cystostomy catheters, and Group B dogs (n=5 ) had both a transurethral catheter and a cystostomy tube placed. All dogs had catheters maintained with a closed urine collection system for seven days. Dogs were observed for ten weeks following surgery, and urinalysis and urine cultures were performed on weeks 1, 4, and 8. Preoperative evaluations were repeated ten weeks postoperatively just prior to termination of the study. Radiographic and histopathologic evaluation of the urethral specimen was performed. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

GLOSSARY of TREATMENT TERMS (Referenced from the Training Manual Prepared by the American College of Surgeons Commission on Cancer)
Appendix 15 GLOSSARY OF TREATMENT TERMS (referenced from the training manual prepared by the American College of Surgeons Commission on Cancer) Abdominal-perineal resection: Surgical procedure used in the treatment of colorectal cancer that requires a combined approach through the abdomen and the perineum. Complications include ureteral injury, urinary dysfunction, urinary tract infections, sexual dysfunction, perineal and abdominal wound infections, and stomal complications. Adjuvant therapy: A therapy that aids another, such as chemotherapy, after surgery. Allogenic bone marrow transplantation: Transplanting bone marrow from one person to another person who is of the same tissue type. Amputation: The removal of a limb or other appendage or outgrowth of the body. Anti-oncogenes: Genes having the ability to regulate growth and inhibit carcinogenesis. Antrectomy: Excision of the antrum. Autologous bone marrow transplatation: Transplanting the patient’s own bone marrow after ablative treatment. BCG: Bacille Calmette Guerin vaccine, a tuberculosis vaccine, containing living, avirulent, bone-strain tubercle bacilli. It is administered by a special technique using a multiple puncture disk and is used in immunotherapy for the treatment of cancer, particularly malignant melanoma and bladder cancer. Bilobectomy: Removal of two lobes. Bilroth I: Pylorectomy with end-to-end anastomosis of the upper portion of the stomach to the duodenum. Bilroth II: Partial gastric resection with closure of duodenal stump and gastrojejunostomy. Biospy: Removal -

1 Annex 2. AHRQ ICD-9 Procedure Codes 0044 PROC-VESSEL
Annex 2. AHRQ ICD-9 Procedure Codes 0044 PROC-VESSEL BIFURCATION OCT06- 0201 LINEAR CRANIECTOMY 0050 IMPL CRT PACEMAKER SYS 0202 ELEVATE SKULL FX FRAGMNT 0051 IMPL CRT DEFIBRILLAT SYS 0203 SKULL FLAP FORMATION 0052 IMP/REP LEAD LF VEN SYS 0204 BONE GRAFT TO SKULL 0053 IMP/REP CRT PACEMAKR GEN 0205 SKULL PLATE INSERTION 0054 IMP/REP CRT DEFIB GENAT 0206 CRANIAL OSTEOPLASTY NEC 0056 INS/REP IMPL SENSOR LEAD OCT06- 0207 SKULL PLATE REMOVAL 0057 IMP/REP SUBCUE CARD DEV OCT06- 0211 SIMPLE SUTURE OF DURA 0061 PERC ANGIO PRECEREB VES (OCT 04) 0212 BRAIN MENINGE REPAIR NEC 0062 PERC ANGIO INTRACRAN VES (OCT 04) 0213 MENINGE VESSEL LIGATION 0066 PTCA OR CORONARY ATHER OCT05- 0214 CHOROID PLEXECTOMY 0070 REV HIP REPL-ACETAB/FEM OCT05- 022 VENTRICULOSTOMY 0071 REV HIP REPL-ACETAB COMP OCT05- 0231 VENTRICL SHUNT-HEAD/NECK 0072 REV HIP REPL-FEM COMP OCT05- 0232 VENTRI SHUNT-CIRCULA SYS 0073 REV HIP REPL-LINER/HEAD OCT05- 0233 VENTRICL SHUNT-THORAX 0074 HIP REPL SURF-METAL/POLY OCT05- 0234 VENTRICL SHUNT-ABDOMEN 0075 HIP REP SURF-METAL/METAL OCT05- 0235 VENTRI SHUNT-UNINARY SYS 0076 HIP REP SURF-CERMC/CERMC OCT05- 0239 OTHER VENTRICULAR SHUNT 0077 HIP REPL SURF-CERMC/POLY OCT06- 0242 REPLACE VENTRICLE SHUNT 0080 REV KNEE REPLACEMT-TOTAL OCT05- 0243 REMOVE VENTRICLE SHUNT 0081 REV KNEE REPL-TIBIA COMP OCT05- 0291 LYSIS CORTICAL ADHESION 0082 REV KNEE REPL-FEMUR COMP OCT05- 0292 BRAIN REPAIR 0083 REV KNEE REPLACE-PATELLA OCT05- 0293 IMPLANT BRAIN STIMULATOR 0084 REV KNEE REPL-TIBIA LIN OCT05- 0294 INSERT/REPLAC SKULL TONG 0085 RESRF HIPTOTAL-ACET/FEM -

Ipo) List for Cy 2021 (N=266)
TABLE 31: PROPOSED MUSCULOSKELETAL-RELATED SERVICE REMOVALS FROM THE INPATIENT ONLY (IPO) LIST FOR CY 2021 (N=266) CY CY 2020 Long Descriptor Related Proposed Proposed 2020 Services CY 2021 CY 2021 CPT OPPS OPPS APC Code Status Assignment Indicator 0095T Removal of total disc arthroplasty 22856 N/A (artificial disc), anterior approach, each additional interspace, cervical (list separately in addition to code for primary procedure) 0098T Revision including replacement 22858 N/A of total disc arthroplasty (artificial disc), anterior approach, each additional interspace, cervical (list separately in addition to code for primary procedure) 0163T Total disc arthroplasty (artificial 22858 N/A disc), anterior approach, including discectomy to prepare interspace (other than for decompression), each additional interspace, lumbar (list separately in addition to code for primary procedure) 0164T Removal of total disc 22856 N/A arthroplasty, (artificial disc), anterior approach, each additional interspace, lumbar (list separately in addition to code for primary procedure) 0165T Revision including replacement 22858 N/A of total disc arthroplasty (artificial disc), anterior approach, each additional interspace, lumbar (list separately in addition to code for primary procedure) 0202T Posterior vertebral joint(s) 63030 J1 5115 arthroplasty (for example, facet joint[s] replacement), including facetectomy, laminectomy, foraminotomy, and vertebral column fixation, injection of bone cement, when performed, including fluoroscopy, single level, lumbar spine