Is Essential for the Development, Survival and Function of Hypoglossal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cranial Nerves
Cranial Nerves (1,5,7,8,9,10,11 and 12) Slides not included 9th and 10th Cranial 11th and 12th Cranial 8th Cranial Nerve 5th and 7th Cranial 1st Cranial Nerve Nerves Nerves Nerves (3,7,11,12,13,21,23,24) - (10,16) (12,23) Slides included: (14 to 17) *Slides that are not included mostly are slides of summaries or pictures. Nouf Alabdulkarim. Med 435 Olfactory Nerve [The 1st Cranial Nerve] Special Sensory Olfactory pathway 1st order neuron Receptors Axons of 1st order Neurons Olfactory receptors are specialized, ciliated nerve cells The axons of these bipolar cells 12 -20 fibers form the that lie in the olfactory epithelium. true olfactory nerve fibers. Which passes through the cribriform plate of ethmoid → They join the olfactory bulb Preliminary processing of olfactory information It is within the olfactory bulb, which contains interneurones and large Mitral cells; axons from the latter leave the bulb to form the olfactory tract. nd 2 order neuron • It is formed by the Mitral cells of olfactory bulb. • The axons of these cells form the olfactory tract. • Each tract divides into 2 roots at the anterior perforated substance: Lateral root Medial root Carries olfactory fibers to end in cortex of the Uncus & • crosses midline through anterior commissure adjacent part of Hippocampal gyrus (center of smell). and joins the uncrossed lateral root of opposite side. • It connects olfactory centers of 2 cerebral hemispheres. • So each olfactory center receives smell sensation from both halves of nasal cavity. NB. Olfactory pathway is the only sensory pathway which reaches the cerebral cortex without passing through the Thalamus . -

The Use of Cholinesterase Techniques to Study Topographical Localization in the Hypoglossal Nucleus of the Rat
J. Anat. (1971), 110, 2, pp. 203-213 203 With 1O figures Printed in Great Britain The use of cholinesterase techniques to study topographical localization in the hypoglossal nucleus of the rat P. R. LEWIS*, B. A. FLUMERFELTt AND C. C. D. SHUTE* Department ofAnatomy, University of Cambridge (Accepted 4 August 1971) INTRODUCTION The hypoglossal nucleus is perhaps the most suitable motor nucleus for the experi- mental study of the cytological changes occurring in cholinergic neurons following axotomy. The cells are large and the nucleus is easy to find even in a fresh unfixed brain; furthermore, the nucleus is so close to the midline that it is possible to use one side as a control for the other with complete confidence and to view equivalent control and experimental neurons simultaneously at quite high magnifications. An added advantage is that the hypoglossal nerve trunk in the neck region is almost purely motor; the central effects of axotomy are therefore not complicated by any significant loss of sensory fibres. Our interest in the nucleus was heightened by the discovery that in the rat a group of neurons at the caudal end contained a high concentration of an enzyme resembling in its histochemical reactions pseudocholin- esterase (Shute & Lewis, 1963). The enzyme will hydrolyse acetylthiocholine and is inhibited by ethopropazine, but its most characteristic property is a rapid hydrolysis of butyrylthiocholine; BuChE would thus seem an appropriate abbreviation to distinguish it from true cholinesterase (AChE), the enzyme typically present in motor neurons. It was shown originally by Schwarzacher (1958) that there is a marked decrease in AChE activity in hypoglossal neurons during the second and third weeks following axotomy (although he also looked at the response of pseudocholinesterase he did not comment on the specifically staining group of cells). -

Hemisus Marmoratum
Neuroscience Letters 244 (1998) 5–8 Distribution of hypoglossal motor neurons innervating the prehensile tongue of the African pig-nosed frog, Hemisus marmoratum Curtis W. Andersona,*, Kiisa C. Nishikawab, Joyce Keifera aDepartment of Anatomy and Structural Biology, University of South Dakota School of Medicine, Vermillion, SD 57069, USA bDepartment of Biological Sciences, Physiology and Functional Morphology Group, Northern Arizona University, Flagstaff, AZ 86011, USA Received 15 December 1997; received in revised form 28 January 1998; accepted 28 January 1998 Abstract Using retrograde neuronal tracers, a study of the distribution of hypoglossal motor neurons innervating the tongue musculature was performed in the African pig-nosed frog, Hemisus marmoratum. This species is a radically divergent anuran amphibian with a prehensile tongue that can be aimed in three dimensions relative to the head. The results illustrate a unique rostrocaudal distribution of the ventrolateral hypoglossal nucleus and an unusually large number of motor neurons within this cell group. During the evolution of the long, prehensile tongue of Hemisus, the motor neurons innervating the tongue have greatly increased in number and have become more caudally distributed in the brainstem and spinal cord compared to other anurans. These observations have implications for understanding neuronal reconfiguring of motoneurons for novel morphologies requiring new muscle activation patterns. 1998 Elsevier Science Ireland Ltd. Keywords: Anuran amphibian; Hypoglossal nerve; Motor nuclei; Tongue Recent studies of feeding in anurans have revealed a hylidae [12,15]. In hydrostatic elongation, the tongue diversity of tongue morphologies and feeding mechanisms protractor muscle (M. genioglossus) has two types of mus- [1,5,7,8,10,11,15]. -
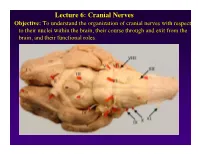
Lecture 6: Cranial Nerves
Lecture 6: Cranial Nerves Objective: To understand the organization of cranial nerves with respect to their nuclei within the brain, their course through and exit from the brain, and their functional roles. Olfactory Eye Muscles 3, 4 &6 Cranial Nerves 1-7 I overview Table, Page 49 II Lecture notes Cranial Nerves and their Functions V Trigeminal VII Facial VIII IX X XII XI Cranial Nerves 8-12 Overview sternocephalic I. Factors Responsible for the Complex Internal Organization of the Brain Stem-> leads to altered location of cranial nerve nuclei in adult brain stem 1. Development of the Fourth Ventricle a. Medulla and Pons develop ventral to the 4th ventricle cerebellum b. Alar plate is displaced lateral to basal plate 4 Medulla Developing Neural Tube 2. Cranial nerve nuclei form discontinuous columns Rostral 12 SE Page 48 Notes 3. Some cranial nerve nuclei migrate from their primitive embryonic positions (e.g., nuclei of V and VII) Facial N. Factors responsible for the complex internal organization of the brainstem: 4) Special senses develop in association with the brain stem. Nuclei of special senses 5) Development of the cerebellum and its connections Cerebellum II. Cranial Nerve Nuclei: Nucleus = column of neuron cell bodies. Efferent nuclei are composed of cell bodies of alpha or gamma motor neurons (SE) or preganglionic parasympathetic neurons (VE). III. Motor Efferent Nuclei (Basal Plate Derivatives): 1. SE (Somatic Efferent) Nuclei: SE neurons form two longitudinally oriented but discontinuous columns of cell bodies in the brain stem. Neurons that comprise these columns are responsible for innervating all of the skeletal musculature of the head. -

Appearance and Transient Expression of Oxytocin Receptors in Fetal, Infant, and Peripubertal -Rat Brain Studied by Autoradiography and Electrophysiology
The Journal of Neuroscience, May 1969, g(5): 1764-I 773 Appearance and Transient Expression of Oxytocin Receptors in Fetal, Infant, and Peripubertal -Rat Brain Studied by Autoradiography and Electrophysiology Eliane Tribollet,’ Serge Charpak,’ Anne Schmidt,* Michel Dubois-Dauphin,’ and Jean Jacques Dreifussl ‘Department of Physiology, University Medical Center, Geneva, Switzerland, and ‘CNRS-INSERM, Center of Pharmacology-Endocrinology, Montpellier, France The development of oxytocin (OT) receptors in the rat brain The classicalhormonal effects of oxytocin (OT) releasedfrom and spinal cord was studied by in vitro light microscopic the hypothalamoneurohypophyseal axons are well established. autoradiography and by electrophysiology. OT receptors were In addition, OT is also present in axons projecting to various labeled using a monoiodinated OT antagonist in tissue sec- areaswithin the CNS (Sofroniew, 1985), which suggeststhat it tions from animals aged between embryonic day 12 (E12) may play a neurotransmitter or neuromodulator role. Addi- and postnatal day 90 (PN90); the response of ongoing spike tional evidence for such a role includes its releaseunder exper- activity to the addition of OT was assessed in neurons lo- imental conditions in situ and in vitro (Buijs, 1983), its effect cated in the dorsal motor nucleus of the vagus nerve of the on the electrical activity of single neurons in the brain (Mtihle- neonate. thaler et al., 1983, 1984; Charpak et al., 1984), the fact that Specific binding was detected first at El4 in a region that small amounts of OT injected into the brain or the cerebral later differentiated into the dorsal motor nucleus of the vagus ventricles modulate neuroendocrine and autonomic functions nerve. -

Differentiation of the Bulbar Motor Nuclei and the Coincident Develop- Ment of Associated Root Fibers in the Rsbbit
DIFFERENTIATION OF THE BULBAR MOTOR NUCLEI AND THE COINCIDENT DEVELOP- MENT OF ASSOCIATED ROOT FIBERS IN THE RSBBIT DONALD L. KIMMEL Department of Anatomy, University of Michigan,’ Aim Arbor THIRTY-ONE FIGURES CONTENTS Introduction ........................................................ 83 Material and methods ................................................ 84 General survey of the literature ....................................... 85 Description of material studied ........................................ 86 Somatic efferent component. Its central and peripheral development .... 86 The hypoglossal ............................................. 86 The abdueens ............................................... 96 Visceral efferent component. Its central and peripheral development .... 103 The vago-accessory ........................................... 103 The glossopharyngeal ........................................ 124 The facial .................................................. 129 The trigemiiial .............................................. 137 Geiicrxldisrussion .................................................... 143 INTRODUCTION This present study concerns itself primarily with the onto- genetic development of the nuclear centers of bulbar cranial nerves in the rabbit and with the embryonic and adult distri- butions of their branches. Its purpose is to show that the central development proceeds stage for stage with the pro- A dissertation submitted in partial fulfillment of the requirements for the degree of doctor of philosophy -

Nuclear Architecture in the Medulla Oblongata of the Adult African Giant Pouched Rat (Cricetomys Gambianus, Waterhouse - 1840)
Int. J. Morphol., 29(2):382-388, 2011. Nuclear Architecture in the Medulla Oblongata of the Adult African Giant Pouched Rat (Cricetomys gambianus, Waterhouse - 1840) Arquitectura Nuclear en la Médula Oblonga de la Rata Gigante de Carillos Africana Adulta (Cricetomys gambianus, Waterhouse - 1840) *Ibe, C. S; *Onyeanusi, B. I.; *Hambolu, J. O. & **Ayo, J. O. IBE, C. S.; ONYEANUSI, B. I.; HAMBOLU, J. O. & AYO, J. O. Nuclear architecture in the medulla oblongata of the adult African giant pouched rat (Cricetomys gambianus, Waterhouse - 1840). Int. J. Morphol., 29(2):382-388, 2011. SUMMARY: The architecture of cranial and non-cranial nerve nuclei in the medulla oblongata of the African giant pouched rat was studied by means of light microscopy. Serial sections of the medulla oblongata, in coronal and saggital planes, were stained with the cresyl fast violet and silver stains, respectively. Sections in the saggital plane were used as a guide, while coronal sections were used to identify the nuclei in the rostrocaudal extent of the medulla oblongata. With the obex serving as the landmark, nuclei rostral and caudal to the obex were delineated. Cranial nerve nuclei whose architecture were defined were the motor nucleus of hypoglossal nerve, motor nucleus of vagus nerve, cochlear nucleus, vestibular nucleus and nucleus ambiguus, while non-cranial nerve nuclei identified were the olivary nucleus, solitary tract nucleus, gracile nucleus, cuneate nucleus, spinal nucleus of trigeminal nerve, motor nucleus of corpus trapezoideum, lateral nucleus of reticular formation and gigantocellular nucleus. The olivary nucleus was the most prominent nucleus, while the solitary tract nucleus was faint, and thus, less developed. -

Brainstem Reflexes Herniation Syndromes (CN IX-XII) Lab 7 March 24, 2021 - Dr
Brainstem Reflexes Herniation Syndromes (CN IX-XII) Lab 7 March 24, 2021 - Dr. Krebs ([email protected]) Objectives: 1. Describe the relationship of the functional anatomy of CN IX - XII and the location of their respective nuclei to a neurological exam which examines the brainstem. 2. Explain the neuroanatomical pathways associated with brainstem reflexes tested in the conscious and unconscious patient. 3. Describe the relationship between the sympathetic and parasympathetic innervation of the eye to the clinical assessment of eye reflexes. 4. Describe the relationship of changes in upper limb posture of unconscious patient to underlying damage to the brainstem. 5. Describe the consequences of herniation syndromes associated with increases in intracranial pressure. Videos for Review: Notes: • For identification of the cranial nerves, use online modules and videos, your atlas and micrographs to locate the nuclei listed. • On the brain and brainstem specimens, locate cranial nerves IX, X, XI and XII. Note the level at which they are attached to the brainstem. ** NOTE: Interactive PDFs are best viewed on desktop/laptop computers - functionality is not reliable on mobile devices ** Design & Artwork: The HIVE (hive.med.ubc.ca) 1 Brainstem Reflexes Herniation Syndromes (CN IX-XII) Lab 7 March 24, 2021 - Dr. Krebs ([email protected]) Glossopharyngeal Nerve (CN IX) Modality Associated Nucleus Function Motor Nucleus ambiguus Motor to stylopharyngeus muscle (SVE) Parasympathetic Inferior salivatory nucleus Stimulation of parotid gland (GVE) Taste Solitary nucleus and tract Taste from posterior 1/3 of tongue (SVA) Somatic Sensory Spinal trigeminal nucleus and General sensation from posterior 1/3 of tongue, (GSA) tract pharynx, external ear/tympanic membrane Visceral Sensory Solitary nucleus and tract Carotid body, gag sensation from oropharynx (GVA) Which foramen does CN IX exit through? Highlight and label the nuclei associated with CN IX in this diagram and show the types of fibres that comprise this peripheral nerve. -

Brain Stem Mechanisms Controlling the Jaw Muscle-Tonus of the Dog*
BRAIN STEM MECHANISMS CONTROLLING THE JAW MUSCLE-TONUS OF THE DOG* YOJIRO KAWAMURA, MASAYA FUNAKOSHI, SHUSAKU TSUKAMOTO AND MITSURU TAKATA** Department of Oral-Physiology, Dental, School, Osaka University Kitaku, Osaka, Japan In the previous paper (1) we clearly demonstrated the distribution of the points in the midbrain and medulla of dogs which responded to depressing of the lower jaw, and the electrophysiological characteristics of this response were also analyzed. That is, regular spontaneous unit discharges (the back- ground activities) which were recorded from and along the mesencephalic trigeminal nucleus and root were remarkably inhibited by elevating the lower jaw and accelerated by depressing the jaw. These responses were obtained by strictly localized punctures in the brainstem, accompanied by a short latency and slow adaptation as shown in goat by Cooper, Daniel and Whitterige (2). These responses were also recorded at points in the trigeminal motor nucleus and lateral reticular formation at the level of the brachium pontis. Conversely, the spontaneous electrical activities of some of the points in the medulla near the hypoglossal root and nucleus were inhibited by depressing the lower jaw. In the present paper the functional correlationships between these mesen- cephalic, pontine and bulbar points were analyzed, and additionally the principal factor inducing this response was determined. METHOD Ten adult dogs, all of them precollicular decerebrate and decerebellate animals, were used. The experimental procedures, the method of fixing the animal's head and to record the brain activity were similar as to those reported in the previous report. But in this experiment, two recording needle micro- electrodes 20ƒÊ at the tip (each of them enclosed in a fine glass capillary) were simultaneously inserted into the brainstem stereotaxically. -

The Hypoglossal Complex of Vertebrates
THE HYPOGLOSSAL COMPLEX OF VERTEBRATES JOHN WALTER BARNARD Departments of Anatomy of the Universitu of Michigan. Ann Arbor. and Georgetown Vniversity, Washington, D. C. TWENTY-FOUR FIQUREB CONTENTS Introduction and material ............................................ 489 Description of the hypoglossal complex ................................ 489 Muscular localization ................................................. 513 Discussion .......................................................... 517 Summary ........................................................... 521 INTRODUCTION AND MATERIAL The present work is a study of the hypoglossal nucleus of vertebrates. In this account an attempt has been made to determine, as far as possible, the significance of the various nuclear subgroupings, and to relate their description with others in the literature. The normal material used in describ- ing the hypoglossal nucleus and its root is part of the Huber Neurological Collection of the Department of Anatomy of the University of Michigan and consists of both cell and fiber preparations of the frog, several turtles, several lizards, the alligator, four birds, the opossum, the shrew, the bat, the mouse, the rat, the rabbit, the sheep, the dog, the cat, the macaque monkey and man. I wish to take this opportunity expressing my appreciation for the assistance and kindly criticism given to me by Doctor Elizabeth Crosby. DESCRIPTION OF THE HYPOGLOSSAL COMPLEX Frog The frog is the lowest vertebrate to have a discrete hypo- glossal nucleus and a muscular tongue. In Rana catesbiana 489 490 JOHN WALTER BARNABD some of the cephalic cells of the cervical motor column have migrated to the dorsolateral aspect of the medial longitudinal fasciculus (fig. 1). These large, coarsely granulated, multi- polar neurons with long dendrites spreading out into the lateral field, make up the dorsomedial iiucleus as described by Black ( '17). -

Brainstem II
Brainstem II Medical Neuroscience Dr. Wiegand Internal Brainstem | Cranial nerve nuclei | Location of selected tracts | Reticular formation Developmental Organization 1 Developmental Organization Sulcus Limitans Developmental Organization FromFrom PritchardPritchard && Alloway:Alloway: Fig.Fig. 4-14-1 2 Cranial Nerve Nuclei Organization | Medial to sulcus limitans z GSE ⇒ SVE ⇒ GVE | Lateral from sulcus limitans z VA ⇒ GSA ⇒ SSA FromFrom PritchardPritchard && Alloway:Alloway: Fig.Fig. 4-44-4 SEN MOT Generalizations | Sensory nuclei lateral to sulcus limitans | Motor nuclei medial to sulcus limitans | Visceral nuclei are on either side of sulcus | Innervation of skeletal muscle (GSE & SVE) most medial | General and special visceral afferent nuclei in same column I, II Cranial Nerves – Telencephalon & Diencephalon | Olfactory – z smell (SVA) | Optic – z vision (SSA) 3 III, IV Cranial Nerves – Mesencephalon | Oculomotor – z extraocular eye muscles (GSE) – oculomotor nucleus z PSNS to eye (GVE) – Edinger-Westphal nucleus | Trochlear – z extraocular muscle (sup. oblique) (GSE) – trochlear nucleus V, VI Cranial Nerves – Metencephalon | Trigeminal – z Masticatory muscles (SVE) – trigeminal motor nucleus z General sensation of the head and face (GSA) – trigeminal complex | Abducens – z extraocular muscle (lat. rectus) (GSE) – abducens nucleus VII Cranial Nerves – Metencephalon | Facial – z Facial expression muscles (SVE) – facial motor nucleus z Glands (submandibular, sublingual & lacrimal) (GVE) – superior salivatory & lacrimal nucleus z Taste (SVA) – rostral solitary nucleus z General sensation of ear (GSA) – trigeminal complex 4 VIII Cranial Nerves – Metencephalon Vestibulocochlear – z Hearing (SSA) – dorsal and ventral cochlear nuclei z Balance (SSA) – vestibular nuclei IX Cranial Nerves – Mylencephalon | Glossopharyngeal z Stylopharyngeus muscle (SVE) – n. ambiguus z PSNS to parotid gland (GVE) – inferior salivatory n. z Taste (SVA) – rostral solitary n. -

A Circumscribed Projection from the Nucleus of the Solitary Tract to The
The Journal of Neuroscience, May 1989, g(5): 1668-l 682 A Circumscribed Projection from the Nucleus of the Solitary Tract to the Nucleus Ambiguus in the Rat: Anatomical Evidence for Somatostatin-284mmunoreactive Interneurons Subserving Reflex Control of Esophageal Motility E. T. Cunningham, Jr.‘s21a and P. E. Sawchenko2 ‘Department of Neurosciences, University of California, San Diego, La Jolla, California 92093, and 2The Salk Institute for Biological Studies and The Clayton Foundation for Research-California Division, La Jolla, California 92037 Axonal transport and immunohistochemical methods were The nucleus of the solitary tract (NTS) occupies a pivotal po- used to investigate the anatomical and biochemical orga- sition in the central visceromotor system. Projections of the nization of projections from the nucleus of the solitary tract Vth, VIIth, IXth, and Xth cranial nerves terminate centrally (NTS) to the rostral, esophageal, part of the nucleus ambig- within the NTS, conveying sensoryinformation from the oral, uus (NA) in the rat. Discrete iontophoretic deposits of a ret- thoracic, and abdominal cavities. The NTS, in turn, projects to rogradely transported tracer, fluorogold, placed in the rostra1 virtually all levels of the neuraxis, so as to coordinate appro- NA labeled a column of cells within the NTS, termed the priate behavioral, neuroendocrine, and autonomic responses central part of the NTS (after Ross et al., 1985) situated just (seeSawchenko, 1983; Norgren, 1984, for reviews). medial to the solitary tract and extending