Spikedace Recovery Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sonora Sucker
scientific name common name Catostomus insignis Sonora sucker Bison code 010520 ______________________________________________________________ Official status Endemism ________________________ State AZ: threatened Colorado River Basin _______________________ Status/threats Dams, diversions, groundwater pumping and introduced species Distribution The species is widespread and abundant in the Gila and Bill Williams river drainages in Arizona and the Gila and San Francisco drainages in southwestern New Mexico. The species is widespread and abundant in the Verde and Gila headwaters. Habitat Streams and rivers from 300 to 3000 m in elevation, primarily in pool habitats. Pool habitats over sand gravel substrates. Life history and ecology Can attain a size of 0.8 m and a weight of greater than 2.0 kg. Used as food by early, primitive human populations. Food habits vary with availability. In one stream, Aravaipa Creek, it is principally a carnivore, whereas elsewhere in pool habitats diet consists of plant debris, mud, and algae. Observed to "suck" cottonwood seeds at surface as is common for the common carp. Young often feed in large schools at stream margins on micro-crustaceans, protozoans and other animal and plant groups. Breeding Similar to most slim-bodied suckers, the species spawns in smaller streams over gravel substrates. Males darken in color and often display extreme tuberculation. Males &(usually 2) flank a single, larger female. Gametes are emitted with considerable to extreme substrate agitation and fall into gravel interstices. Cleaning of gravels occurs much as reported for salmonid species. Key Habitat Components: pools with sand-gravel substrates for adults and shallow, low velocity riffles and backwaters for young Breeding season Protracted, from as early as January to February at low elevations to as late as July. -

Edna Assay Development
Environmental DNA assays available for species detection via qPCR analysis at the U.S.D.A Forest Service National Genomics Center for Wildlife and Fish Conservation (NGC). Asterisks indicate the assay was designed at the NGC. This list was last updated in June 2021 and is subject to change. Please contact [email protected] with questions. Family Species Common name Ready for use? Mustelidae Martes americana, Martes caurina American and Pacific marten* Y Castoridae Castor canadensis American beaver Y Ranidae Lithobates catesbeianus American bullfrog Y Cinclidae Cinclus mexicanus American dipper* N Anguillidae Anguilla rostrata American eel Y Soricidae Sorex palustris American water shrew* N Salmonidae Oncorhynchus clarkii ssp Any cutthroat trout* N Petromyzontidae Lampetra spp. Any Lampetra* Y Salmonidae Salmonidae Any salmonid* Y Cottidae Cottidae Any sculpin* Y Salmonidae Thymallus arcticus Arctic grayling* Y Cyrenidae Corbicula fluminea Asian clam* N Salmonidae Salmo salar Atlantic Salmon Y Lymnaeidae Radix auricularia Big-eared radix* N Cyprinidae Mylopharyngodon piceus Black carp N Ictaluridae Ameiurus melas Black Bullhead* N Catostomidae Cycleptus elongatus Blue Sucker* N Cichlidae Oreochromis aureus Blue tilapia* N Catostomidae Catostomus discobolus Bluehead sucker* N Catostomidae Catostomus virescens Bluehead sucker* Y Felidae Lynx rufus Bobcat* Y Hylidae Pseudocris maculata Boreal chorus frog N Hydrocharitaceae Egeria densa Brazilian elodea N Salmonidae Salvelinus fontinalis Brook trout* Y Colubridae Boiga irregularis Brown tree snake* -

LITTLE COLORADO RIVER SPINEDACE, Lepidomeda Vitata RECOVERY PLAN
DRAFT LITTLE COLORADO RIVER SPINEDACE, Lepidomeda vitata RECOVERY PLAN prepared by: C.O. Minckley U.S. Fish and Wildlife Service, Region 2 Parker Fishery Resource Office, Parker, Arizona 85344 August 1994 for U.S. Fish and Wildlife Service, Albuquerque, New Mexico ' DISCLAIMER Recovery plans delineate reasonable actions which are believed to be required to recover and/or protect listed species. Plans are published by the U.S. Fish and Wildlife Service, sometimes prepared with the assistance of recovery teams, contractors, State agencies, and others. Objectives will be attained and any necessary funds made available, subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. Recovery plans do not necessarily represent the views nor the official positions or approvals of any individuals or agencies (involved in the plan formulation), other than the U.S. Fish and Wildlife Service. They represent the official position of the U.S. Fish and Wildlife Service only after they have been signed by the Regional Director or Director as approved. Approved recovery plans are subject to modification as dictated by new findings, changes in species status, and the completion of recovery tasks. ...) i ' ,4 , ' P DRAF1- ig l Li &a-liATION8 U.S. Fish and Wildlife Service. 199. Little Colorado River spinedace, Lepidomeda vittata Recovery Plan. Phoenix, AZ pp. Additional copies may be purchased from: Fish and Wildlife Reference Service: 5430 Grosvenor Lane, Suite 110 Bethesda, Maryland 20814 301/492-6403 or 1-800-582-3421 The fee for the Plan varies depending on the number of pages of the Plan. -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Laughing Waters" 40
Press, W. H., B. P. Flannery, S. A. Teukolsky, and W. T. Vetterling. 1986. Numerical recipes: C 1990 by S.E.L & Associates The art of scientific computing. Cambridge, MA: Cambridge University Press. Stalnaker, C. B. 1978. The IFG incremental methodology for physical instream habitat eval- uation. Washington, DC: U.S. Fish and Wildlife Service (FWS / OBS-78 /81). Stier, D. J., and J. H. Crance. 1985. Habitat suitability index models and instream flow suitability curves: American shad. Washington, DC: U.S. Fish and Wildlife Service (FWS/ OBS-82 /10). The Recreational Impact of Trihey, E. W. 1979. The IFG incremental methodology. Pages 24-44 in G. L. Smith, editor. Proceedings of the workshop in instream flow habitat criteria and modeling. Information Series Reducing the "Laughing Waters" 40. Fort Collins: Colorado State University, Colorado Water Resources Research Institute. Valdez, R. A., and B. C. Nilson. 1982. Radiotelemetry as a means of assessing movement and of Aravaipa Creek, Arizona habitat selection of humpback chub. Pages 29-39 in W. Geer, editor. Transactions of the Bonneville Chapter of the American Fisheries Society. Salt Lake City, UT: Bonneville Chapter of the American Fisheries Society. Steven D. Moore Valdez, R. A., P. B. Holden, T. B. Hardy, and R. J. Ryel. 1987. Habitat suitability index curves Mary E. Wilkosz* for endangered fishes of the Upper Colorado River Basin. Final report to US. Fish and Wildlife Service (Contract No. 14-16-0006-86-055), Denver, CO. Stanley K. Brickler Received: May 3, 1989 School of Renewable Natural Resources Accepted: June 25, 1989 University of Arizona Discussion open until August 1, 1990 Tucson, Arizona 85721 ABSTRACT: The paper describes analyses that were conducted to de- termine the importance of water as an attribute of the recreational setting at Aravaipa Canyon Wilderness, Arizona, and the influence that reduced flows in Aravaipa Creek would have on visitors' perceptions of water quantity and quality. -

Aravaipa Canyon Ecosystem Management Plan
BLM Aravaipa Ecosystem Management Plan Final Aravaipa and Environmental Assessment Ecosystem Management Plan and Environmental Assessment Arizona • Gila District • Safford Field Office Field • Safford •District Gila Arizona September 2015 i April 2015 Mission Statements Bureau of Land Management The Bureau of Land Management (BLM) is responsible for managing the National System of Public Lands and its resources in a combination of ways, which best serves the needs of the American people. The BLM balances recreational, commercial, scientific and cultural interests and it strives for long-term protection of renewable and nonrenewable resources, including range, timber, minerals, recreation, watershed, fish and wildlife, wilderness and natural, scenic, scientific and cultural values. It is the mission of the BLM to sustain the health, diversity and productivity of the public lands for the use and enjoyment of present and future generations. Arizona Game and Fish Department The mission of the Arizona Game and Fish Department is to conserve Arizona’s diverse wildlife resources and manage for safe, compatible outdoor recreation opportunities for current and future generations. The Nature Conservancy The mission of The Nature Conservancy is to preserve the plants, animals and natural communities that represent the diversity of life on Earth by protecting the lands and waters they need to survive. Cover photo: Aravaipa Creek. Photo © Greg Gamble/TNC BLM/AZ/PL-08/006 ii United States Department of the Interior BUREAU OF LAND MANAGEMENT Safford Field Office 711 South 14th Avenue, Suite A Safford, Arizona 8 5546~3335 www.blm.gov/azl September 15, 2015 In Reply Refer To: 8372 (0010) Dear Reader: The document accompanying this letter contains the Final Aravaipa Ecosystem Management Plan, Environmental Assessment, Finding ofNo Significant Impact, and Decision Record. -

Parker Little Colorado River Spinedace Recovery Plan
Parker Fisheries Resource Office Little Colorado River Spinedace Recovery Plan Little Colorado River Spinedace Lepidomeda vittata Recovery Plan prepared by: C. 0. Minckley U.S. Fish and Wildlife Service Parker Fishery Resource Office, Parker, Arizona 85344 October 1997 for U.S. Fish and Wildlife Service, Albuquerque, New Mexico Approved: ~~~ector Regi Date: r~IAN 09 1998 Little Colorado River Spinedace Recovery Plan DISCLAIMER Recovery plans delineate reasonable actions believed necessary to recover and/or protect listed species. Plans are published by the U.S. Fish and Wildlife Service, sometimes prepared with the assistance of recovery teams, contractors, State agencies, and others. Objectives will be attained and any necessary funds made available, subject to budgetary and other constraints affecting the stakeholders involved, as well as the need to address other priorities. Recovery plans do not necessarily represent the views nor the official positions or approvals ofany individuals or agencies (involved in the plan formulation), other than the U.S. Fish and Wildlife Service. They represent the official position ofthe U.S. Fish and Wildlife Service only after they have been signed by the Regional Director or Director as approved. Approved recovery plans are subject to modification as dictated by new findings, changes in species status, and the completion ofrecovery tasks. Little Colorado River Spinedace Recovery Plan LITERATURE CITATION U.S. Fish and Wildlife Service. 1998. Little Colorado River spinedace, Lepido-meda vittata Recovery Plan. Albuquerque NM. 51 pp. Additional copies may be purchased from: Fish and Wildlife Reference Service: 5430 Grosvenor Lane, Suite 110 Bethesda, Maryland 20814 301/492-6403 or 800/582-3421 Fees charged for Recovery Plans vary depending on the number of Plans requested. -
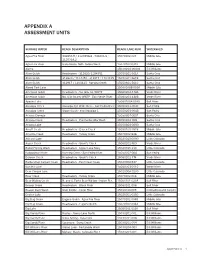
Appendix a Assessment Units
APPENDIX A ASSESSMENT UNITS SURFACE WATER REACH DESCRIPTION REACH/LAKE NUM WATERSHED Agua Fria River 341853.9 / 1120358.6 - 341804.8 / 15070102-023 Middle Gila 1120319.2 Agua Fria River State Route 169 - Yarber Wash 15070102-031B Middle Gila Alamo 15030204-0040A Bill Williams Alum Gulch Headwaters - 312820/1104351 15050301-561A Santa Cruz Alum Gulch 312820 / 1104351 - 312917 / 1104425 15050301-561B Santa Cruz Alum Gulch 312917 / 1104425 - Sonoita Creek 15050301-561C Santa Cruz Alvord Park Lake 15060106B-0050 Middle Gila American Gulch Headwaters - No. Gila Co. WWTP 15060203-448A Verde River American Gulch No. Gila County WWTP - East Verde River 15060203-448B Verde River Apache Lake 15060106A-0070 Salt River Aravaipa Creek Aravaipa Cyn Wilderness - San Pedro River 15050203-004C San Pedro Aravaipa Creek Stowe Gulch - end Aravaipa C 15050203-004B San Pedro Arivaca Cienega 15050304-0001 Santa Cruz Arivaca Creek Headwaters - Puertocito/Alta Wash 15050304-008 Santa Cruz Arivaca Lake 15050304-0080 Santa Cruz Arnett Creek Headwaters - Queen Creek 15050100-1818 Middle Gila Arrastra Creek Headwaters - Turkey Creek 15070102-848 Middle Gila Ashurst Lake 15020015-0090 Little Colorado Aspen Creek Headwaters - Granite Creek 15060202-769 Verde River Babbit Spring Wash Headwaters - Upper Lake Mary 15020015-210 Little Colorado Babocomari River Banning Creek - San Pedro River 15050202-004 San Pedro Bannon Creek Headwaters - Granite Creek 15060202-774 Verde River Barbershop Canyon Creek Headwaters - East Clear Creek 15020008-537 Little Colorado Bartlett Lake 15060203-0110 Verde River Bear Canyon Lake 15020008-0130 Little Colorado Bear Creek Headwaters - Turkey Creek 15070102-046 Middle Gila Bear Wallow Creek N. and S. Forks Bear Wallow - Indian Res. -

Fishtraits: a Database on Ecological and Life-History Traits of Freshwater
FishTraits database Traits References Allen, D. M., W. S. Johnson, and V. Ogburn-Matthews. 1995. Trophic relationships and seasonal utilization of saltmarsh creeks by zooplanktivorous fishes. Environmental Biology of Fishes 42(1)37-50. [multiple species] Anderson, K. A., P. M. Rosenblum, and B. G. Whiteside. 1998. Controlled spawning of Longnose darters. The Progressive Fish-Culturist 60:137-145. [678] Barber, W. E., D. C. Williams, and W. L. Minckley. 1970. Biology of the Gila Spikedace, Meda fulgida, in Arizona. Copeia 1970(1):9-18. [485] Becker, G. C. 1983. Fishes of Wisconsin. University of Wisconsin Press, Madison, WI. Belk, M. C., J. B. Johnson, K. W. Wilson, M. E. Smith, and D. D. Houston. 2005. Variation in intrinsic individual growth rate among populations of leatherside chub (Snyderichthys copei Jordan & Gilbert): adaptation to temperature or length of growing season? Ecology of Freshwater Fish 14:177-184. [349] Bonner, T. H., J. M. Watson, and C. S. Williams. 2006. Threatened fishes of the world: Cyprinella proserpina Girard, 1857 (Cyprinidae). Environmental Biology of Fishes. In Press. [133] Bonnevier, K., K. Lindstrom, and C. St. Mary. 2003. Parental care and mate attraction in the Florida flagfish, Jordanella floridae. Behavorial Ecology and Sociobiology 53:358-363. [410] Bortone, S. A. 1989. Notropis melanostomus, a new speices of Cyprinid fish from the Blackwater-Yellow River drainage of northwest Florida. Copeia 1989(3):737-741. [575] Boschung, H.T., and R. L. Mayden. 2004. Fishes of Alabama. Smithsonian Books, Washington. [multiple species] 1 FishTraits database Breder, C. M., and D. E. Rosen. 1966. Modes of reproduction in fishes. -

Common Name and the Probability of Occurrence
DEVELOPING PREDICTED DISTRIBUTION MODELS FOR FISH SPECIES IN NEBRASKA Final Report: July 2006 Submitted to: The USGS National Gap Analysis Program Moscow, Idaho Project Number: 00005007 DEVELOPING PREDICTED DISTRIBUTION MODELS FOR FISH SPECIES IN NEBRASKA FINAL REPORT 11 July 2006 Scott P. Sowa Principal Investigator Gust M. Annis NHD Classification, Watershed Characterization, and Distribution Modeling Michael E. Morey Range Mapping and Distribution Modeling Aaron J. Garringer Habitat Affinity Reports Missouri Resource Assessment Partnership School of Natural Resources University of Missouri Columbia, MO 65201 Contract Administration Through: Office of Sponsored Programs University of Missouri-Columbia Submitted by: Scott P. Sowa, PhD Research Performed Under: Cooperative Agreement No. 01-HQ-AG-0202 Cooperative Agreement No. 04-HQ-AG-0134 TABLE OF CONTENTS Abstract.................................................................................................................ii Acknowledgements.............................................................................................iii Introduction..........................................................................................................1 Methods................................................................................................................1 Community Fish Sampling Data..................................................................1 Mapping Geographic Ranges......................................................................2 GIS Base Layer for -

Spatiotemporal Response of Aquatic Native and Nonnative Taxa to Wildfire Disturbance in a Desert Stream Network
SPATIOTEMPORAL RESPONSE OF AQUATIC NATIVE AND NONNATIVE TAXA TO WILDFIRE DISTURBANCE IN A DESERT STREAM NETWORK by JAMES E. WHITNEY B.S., Emporia State University, 2007 M.S., Kansas State University, 2010 AN ABSTRACT OF A DISSERTATION submitted in partial fulfillment of the requirements for the degree DOCTOR OF PHILOSOPHY Division of Biology College of Arts and Sciences KANSAS STATE UNIVERSITY Manhattan, Kansas 2014 Abstract Many native freshwater animals are imperiled as a result of habitat alteration, species introductions and climate-moderated changes in disturbance regimes. Native conservation and nonnative species management could benefit from greater understanding of critical factors promoting or inhibiting native and nonnative success in the absence of human-caused ecosystem change. The objectives of this dissertation were to (1) explain spatiotemporal patterns of native and nonnative success, (2) describe native and nonnative response to uncharacteristic wildfire disturbance, and (3) test the hypothesis that wildfire disturbance has differential effects on native and nonnative species. This research was conducted across six sites in three reaches (tributary, canyon, and valley) of the unfragmented and largely-unmodified upper Gila River Basin of southwestern New Mexico. Secondary production was measured to quantify success of native and nonnative fishes prior to wildfires during 2008-2011. Native fish production was greater than nonnatives across a range of environmental conditions, although nonnative fish, tadpole, and crayfish production could approach or exceed that of native macroinvertebrates and fishes in canyon habitats, a warmwater tributary, or in valley sites, respectively. The second objective was accomplished by measuring biomass changes of a warmwater native and nonnative community during 2010-2013 before and after consecutive, uncharacteristic wildfires. -

Upper Gila Watershed (HUC8 15040001)
Upper Gila Watershed (HUC8 15040001) Rapid Watershed Assessment Upper Gila Watershed 1 Upper Gila Watershed (HUC8 15040001) The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or a part of an individual's income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA's TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination writ e to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, D.C. 20250-9410 or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer. 2 Upper Gila Watershed (HUC8 15040001) Table of Contents Overview ......................................................................................................................................... 5 Physical Setting ............................................................................................................................... 7 Precipitation .................................................................................................................................. 10 Land