Developmental Plasticity and the Origin of Tetrapods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Wingtips of the Pterosaurs: Anatomy, Aeronautical Function and Palaeogeography, Palaeoclimatology, Palaeoecology Xxx (2015) Xxx Xxx 3 Ecological Implications
Our reference: PALAEO 7445 P-authorquery-v11 AUTHOR QUERY FORM Journal: PALAEO Please e-mail your responses and any corrections to: Article Number: 7445 E-mail: [email protected] Dear Author, Please check your proof carefully and mark all corrections at the appropriate place in the proof (e.g., by using on-screen annotation in the PDF file) or compile them in a separate list. Note: if you opt to annotate the file with software other than Adobe Reader then please also highlight the appropriate place in the PDF file. To ensure fast publication of your paper please return your corrections within 48 hours. For correction or revision of any artwork, please consult http://www.elsevier.com/artworkinstructions. We were unable to process your file(s) fully electronically and have proceeded by Scanning (parts of) your Rekeying (parts of) your article Scanning the article artwork Any queries or remarks that have arisen during the processing of your manuscript are listed below and highlighted by flags in the proof. Click on the ‘Q’ link to go to the location in the proof. Location in article Query / Remark: click on the Q link to go Please insert your reply or correction at the corresponding line in the proof Q1 Your article is registered as a regular item and is being processed for inclusion in a regular issue of the journal. If this is NOT correct and your article belongs to a Special Issue/Collection please contact [email protected] immediately prior to returning your corrections. Q2 Please confirm that given names and surnames have been identified correctly. -

Present Status of Fish Biodiversity and Abundance in Shiba River, Bangladesh
Univ. J. zool. Rajshahi. Univ. Vol. 35, 2016, pp. 7-15 ISSN 1023-6104 http://journals.sfu.ca/bd/index.php/UJZRU © Rajshahi University Zoological Society Present status of fish biodiversity and abundance in Shiba river, Bangladesh D.A. Khanom, T Khatun, M.A.S. Jewel*, M.D. Hossain and M.M. Rahman Department of Fisheries, University of Rajshahi, Rajshahi 6205, Bangladesh Abstract: The study was conducted to investigate the abundance and present status of fish biodiversity in the Shiba river at Tanore Upazila of Rajshahi district, Bangladesh. The study was conducted from November, 2016 to February, 2017. A total of 30 species of fishes were recorded belonging to nine orders, 15 families and 26 genera. Cypriniformes and Siluriformes were the most diversified groups in terms of species. Among 30 species, nine species under the order Cypriniformes, nine species of Siluriformes, five species of Perciformes, two species of Channiformes, two species of Mastacembeliformes, one species of Beloniformes, one species of Clupeiformes, one species of Osteoglossiformes and one species of Decapoda, Crustacea were found. Machrobrachium lamarrei of the family Palaemonidae under Decapoda order was the most dominant species contributing 26.29% of the total catch. In the Shiba river only 6.65% threatened fish species were found, and among them 1.57% were endangered and 4.96% were vulnerable. The mean values of Shannon-Weaver diversity (H), Margalef’s richness (D) and Pielou’s (e) evenness were found as 1.86, 2.22 and 0.74, respectively. Relationship between Shannon-Weaver diversity index (H) and pollution indicates the river as light to moderate polluted. -

BONY FISHES 602 Bony Fishes
click for previous page BONY FISHES 602 Bony Fishes GENERAL REMARKS by K.E. Carpenter, Old Dominion University, Virginia, USA ony fishes constitute the bulk, by far, of both the diversity and total landings of marine organisms encoun- Btered in fisheries of the Western Central Atlantic.They are found in all macrofaunal marine and estuarine habitats and exhibit a lavish array of adaptations to these environments. This extreme diversity of form and taxa presents an exceptional challenge for identification. There are 30 orders and 269 families of bony fishes presented in this guide, representing all families known from the area. Each order and family presents a unique suite of taxonomic problems and relevant characters. The purpose of this preliminary section on technical terms and guide to orders and families is to serve as an introduction and initial identification guide to this taxonomic diversity. It should also serve as a general reference for those features most commonly used in identification of bony fishes throughout the remaining volumes. However, I cannot begin to introduce the many facets of fish biology relevant to understanding the diversity of fishes in a few pages. For this, the reader is directed to one of the several general texts on fish biology such as the ones by Bond (1996), Moyle and Cech (1996), and Helfman et al.(1997) listed below. A general introduction to the fisheries of bony fishes in this region is given in the introduction to these volumes. Taxonomic details relevant to a specific family are explained under each of the appropriate family sections. The classification of bony fishes continues to transform as our knowledge of their evolutionary relationships improves. -

Fish Locomotion: Recent Advances and New Directions
MA07CH22-Lauder ARI 6 November 2014 13:40 Fish Locomotion: Recent Advances and New Directions George V. Lauder Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts 02138; email: [email protected] Annu. Rev. Mar. Sci. 2015. 7:521–45 Keywords First published online as a Review in Advance on swimming, kinematics, hydrodynamics, robotics September 19, 2014 The Annual Review of Marine Science is online at Abstract marine.annualreviews.org Access provided by Harvard University on 01/07/15. For personal use only. Research on fish locomotion has expanded greatly in recent years as new This article’s doi: approaches have been brought to bear on a classical field of study. Detailed Annu. Rev. Marine. Sci. 2015.7:521-545. Downloaded from www.annualreviews.org 10.1146/annurev-marine-010814-015614 analyses of patterns of body and fin motion and the effects of these move- Copyright c 2015 by Annual Reviews. ments on water flow patterns have helped scientists understand the causes All rights reserved and effects of hydrodynamic patterns produced by swimming fish. Recent developments include the study of the center-of-mass motion of swimming fish and the use of volumetric imaging systems that allow three-dimensional instantaneous snapshots of wake flow patterns. The large numbers of swim- ming fish in the oceans and the vorticity present in fin and body wakes sup- port the hypothesis that fish contribute significantly to the mixing of ocean waters. New developments in fish robotics have enhanced understanding of the physical principles underlying aquatic propulsion and allowed intriguing biological features, such as the structure of shark skin, to be studied in detail. -

Median Fin Patterning in Bony Fish: Caspase-3 Role in Fin Fold Reabsorption
Eastern Illinois University The Keep Undergraduate Honors Theses Honors College 2017 Median Fin Patterning in Bony Fish: Caspase-3 Role in Fin Fold Reabsorption Kaitlyn Ann Hammock Follow this and additional works at: https://thekeep.eiu.edu/honors_theses Part of the Animal Sciences Commons Median fin patterning in bony fish: caspase-3 role in fin fold reabsorption BY Kaitlyn Ann Hammock UNDERGRADUATE THESIS Submitted in partial fulfillment of the requirement for obtaining UNDERGRADUATE DEPARTMENTAL HONORS Department of Biological Sciences along with the HonorsCollege at EASTERN ILLINOIS UNIVERSITY Charleston, Illinois 2017 I hereby recommend this thesis to be accepted as fulfilling the thesis requirement for obtaining Undergraduate Departmental Honors Date '.fHESIS ADVI 1 Date HONORSCOORDmATOR f C I//' ' / ·12 1' J Date, , DEPARTME TCHAIR Abstract Fish larvae develop a fin fold that will later be replaced by the median fins. I hypothesize that finfold reabsorption is part of the initial patterning of the median fins,and that caspase-3, an apoptosis marker, will be expressed in the fin fold during reabsorption. I analyzed time series of larvae in the first20-days post hatch (dph) to determine timing of median findevelopment in a basal bony fish- sturgeon- and in zebrafish, a derived bony fish. I am expecting the general activation pathway to be conserved in both fishesbut, the timing and location of cell death to differ.The dorsal fin foldis the firstto be reabsorbed in the sturgeon starting at 2 dph and rays formed at 6dph. This was closely followed by the anal finat 3 dph, rays at 9 dph and only later, at 6dph, does the caudal fin start forming and rays at 14 dph. -

Amphibious Fishes: Terrestrial Locomotion, Performance, Orientation, and Behaviors from an Applied Perspective by Noah R
AMPHIBIOUS FISHES: TERRESTRIAL LOCOMOTION, PERFORMANCE, ORIENTATION, AND BEHAVIORS FROM AN APPLIED PERSPECTIVE BY NOAH R. BRESSMAN A Dissertation Submitted to the Graduate Faculty of WAKE FOREST UNIVESITY GRADUATE SCHOOL OF ARTS AND SCIENCES in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY Biology May 2020 Winston-Salem, North Carolina Approved By: Miriam A. Ashley-Ross, Ph.D., Advisor Alice C. Gibb, Ph.D., Chair T. Michael Anderson, Ph.D. Bill Conner, Ph.D. Glen Mars, Ph.D. ACKNOWLEDGEMENTS I would like to thank my adviser Dr. Miriam Ashley-Ross for mentoring me and providing all of her support throughout my doctoral program. I would also like to thank the rest of my committee – Drs. T. Michael Anderson, Glen Marrs, Alice Gibb, and Bill Conner – for teaching me new skills and supporting me along the way. My dissertation research would not have been possible without the help of my collaborators, Drs. Jeff Hill, Joe Love, and Ben Perlman. Additionally, I am very appreciative of the many undergraduate and high school students who helped me collect and analyze data – Mark Simms, Tyler King, Caroline Horne, John Crumpler, John S. Gallen, Emily Lovern, Samir Lalani, Rob Sheppard, Cal Morrison, Imoh Udoh, Harrison McCamy, Laura Miron, and Amaya Pitts. I would like to thank my fellow graduate student labmates – Francesca Giammona, Dan O’Donnell, MC Regan, and Christine Vega – for their support and helping me flesh out ideas. I am appreciative of Dr. Ryan Earley, Dr. Bruce Turner, Allison Durland Donahou, Mary Groves, Tim Groves, Maryland Department of Natural Resources, UF Tropical Aquaculture Lab for providing fish, animal care, and lab space throughout my doctoral research. -

Lecture 6 – Integument ‐ Scale • a Scale Is a Small Rigid Plate That Grows out of an Animal’ S Skin to Provide Protection
Lecture 6 – Integument ‐ Scale • A scale is a small rigid plate that grows out of an animal’s skin to provide protection. • Scales are quite common and have evolved multiple times with varying structure and function. • Scales are generally classified as part of an organism's integumentary system. • There are various types of scales according to shape and to class of animal. • Although the meat and organs of some species of fish are edible by humans, the scales are usually not eaten. Scale structure • Fish scales Fish scales are dermally derived, specifically in the mesoderm. This fact distinguishes them from reptile scales paleontologically. Genetically, the same genes involved in tooth and hair development in mammals are also involved in scale development. Earliest scales – heavily armoured thought to be like Chondrichthyans • Fossil fishes • ion reservoir • osmotic control • protection • Weighting Scale function • Primary function is protection (armor plating) • Hydrodynamics Scales are composed of four basic compounds: ((gmoving from inside to outside in that order) • Lamellar bone • Vascular or spongy bone • Dentine (dermis) and is always associated with enamel. • Acellular enamel (epidermis) • The scales of fish lie in pockets in the dermis and are embeded in connective tissue. • Scales do not stick out of a fish but are covered by the Epithelial layer. • The scales overlap and so form a protective flexible armor capable of withstanding blows and bumping. • In some catfishes and seahorses, scales are replaced by bony plates. • In some other species there are no scales at all. Evolution of scales Placoid scale – (Chondricthyes – cartilagenous fishes) develop in dermis but protrude through epidermis. -

Blackchin Tilapia (Sarotherodon Melanotheron) Ecological Risk Screening Summary
U.S. Fish and Wildlife Service Blackchin Tilapia (Sarotherodon melanotheron) Ecological Risk Screening Summary Web Version – 10/01/2012 Photo: © U.S. Geological Survey From Nico and Neilson (2014). 1 Native Range and Nonindigenous Occurrences Native Range From Nico and Neilson (2014): “Tropical Africa. Brackish estuaries and lagoons from Senegal to Zaire (Trewavas 1983).” Nonindigenous Occurrences From Nico and Neilson (2014): “Established in Florida and Hawaii. Evidence indicates it is spreading rapidly in both fresh and salt water around island of Oahu, Hawaii (Devick 1991b).” “The first documented occurrence of this species in Florida was a specimen gillnetted by commercial fishermen in Hillsborough Bay near Tampa, Hillsborough County, in 1959 (Springer and Finucane 1963). Additional records for the western part of the state indicate that this species is established in brackish and freshwaters in eastern Tampa Bay and in adjoining drainages in Hillsborough County, ranging from the Alafia River south to Cockroach Bay. The species has been recorded from the Alafia River from its mouth up to Lithia Springs; from the Hillsborough River, Bullfrog Creek, the Palm River, and the Little Manatee River; and from various western drainage and irrigation ditches (Springer and Finucane 1963; Finucane and Rinckey 1967; Buntz Sarotherodon melanotheron Ecological Risk Screening Summary U.S. Fish and Wildlife Service – Web Version – 10/01/2012 and Manooch 1969; Lachner et al. 1970; Courtenay et al. 1974; Courtenay and Hensley 1979; Courtenay and Kohler 1986; Lee et al. 1980 et seq.; Courtenay and Stauffer 1990; DNR collections; UF museum specimens). There are two records of this species from the west side of Tampa Bay, in Pinellas County: a collection from Lake Maggiore in St. -

Human Functional Anatomy 213 the Upper Limb Early Limb Development
2 HUMAN FUNCTIONAL ANATOMY 213 THE UPPER LIMB EARLY LIMB DEVELOPMENT THIS WEEKS LAB: IN THE FISH (or early human embryo) Proximal parts, plexuses and patterns Slight elevations of ectoderm appear in lateral plate (4th week). Apical ectodermal ridge induces proliferation of limb mesenchyme. READINGS Dorsal and ventral muscle masses connect the girdle to the limb bud. Stern. Essentials of Gross anatomy – The upper limb Limb girdle in body wall Stern. Core concepts in Anatomy:- 80: Organization of upper limb musculature and the Proximal, middle and distal segments of the limb brachial plexus Faiz and Moffat. Anatomy at a Glance:- Nerves of the Upper limb 1 & 2 (parts 30 &31) Grant's Method:- Upper limb and Back (especially pectoral region and axilla) OR any other regional textbook - similar sections IN THIS LECTURE I WILL COVER: Ontogeny and Phylogeny The Pectoral fin The primitive tetrapod forelimb Rotations of the limb in phylogeny Dorsal and ventral muscle/nerve/girdle bone Segmental nerve supply and muscle groups Brachial plexus Muscle groups of the upper limb The fin, or paddle has: Preaxial and postaxial borders (front and back edges) Dorsal and ventral surfaces (top and bottom) Dorsal muscles elevate the fin. Attach to dorsal elements of the girdle (“scapula” and vertebrae) Ventral muscles depress the fin. Attach to ventral elements of the girdle (coracoid) 3 4 PRIMITIVE TETRAPOD FORELIMB MAMMALIAN FORELIMB ROTATIONS 90 degrees LATERAL ROTATION The characteristic segments of the limb (shoulder, arm, forearm, & hand) Were present in -

Southampton French Quarter 1382 Specialist Report Download E2: Fish Bone
Southampton French Quarter SOU1382 Specialist Report Download E2 Southampton French Quarter 1382 Specialist Report Download E2: Fish Bone By Rebecca Nicholson Introduction An assemblage of almost 7500 identifiable fish bones was recovered both by hand retrieval during the excavation, but predominantly from the sorted residues of the processed bulk soil samples. During excavations at Southampton French Quarter a total of 188 bulk samples were sieved to 0.5mm (occasionally 1mm) as part of the flotation process for the recovery of plant and animal remains. The sampling strategy followed during the excavation involved, where possible, the full sampling of one rubbish pit and one latrine pit per tenement for each major period, avoiding intercut features or those clearly containing residual material. Occupation surfaces and other distinct features such as hearths were also sampled. Mixed contexts or contexts of uncertain provenance were avoided. While complete standardisation of sample volumes was not possible, wherever practicable samples were 40 litres. Following assessment of the fish remains recovered largely from the > 4mm residues, the richer assemblages were targeted for further fine residue and flot sorting. This report comprises an analysis of all the identified the fish remains from these samples, together with the material collected by hand on site. Methodology The residues from all of the bulk-sieved samples were sorted to 4mm. Where samples were identified as having significant numbers of fish bones, residues were sorted to 2mm. Samples from the Late Saxon deposits which produced fish remains were routinely sorted to 2mm even where fish remains were not abundant, in order to avoid a perceived bias against the recovery of small fish in pre-medieval deposits (see Barrett et al. -

Copyright Warning & Restrictions
Copyright Warning & Restrictions The copyright law of the United States (Title 17, United States Code) governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the photocopy or reproduction is not to be “used for any purpose other than private study, scholarship, or research.” If a, user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use” that user may be liable for copyright infringement, This institution reserves the right to refuse to accept a copying order if, in its judgment, fulfillment of the order would involve violation of copyright law. Please Note: The author retains the copyright while the New Jersey Institute of Technology reserves the right to distribute this thesis or dissertation Printing note: If you do not wish to print this page, then select “Pages from: first page # to: last page #” on the print dialog screen The Van Houten library has removed some of the personal information and all signatures from the approval page and biographical sketches of theses and dissertations in order to protect the identity of NJIT graduates and faculty. ABSTRACT THESE FISH WERE MADE FOR WALKING: MORPHOLOGY AND WALKING KINEMATICS IN BALITORID LOACHES by Callie Hendricks Crawford Terrestrial excursions have been observed in multiple lineages of marine and freshwater fishes. These ventures into the terrestrial environment may be used when fish are searching out new habitat during drought, escaping predation, laying eggs, or seeking food sources. -
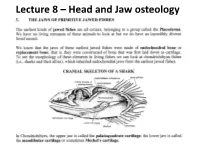
Lecture 8 – Head and Jaw Osteology
Lecture 8 – Head and Jaw osteology More derived fishes (Ray finned fishes) The variability of the jaw structure of bony fishes provides an explanation for the extensive adaptive radiation in the group and why they are so diverse and occupy almost every aquatic niche available. Skull diversity (A) carp, Cyprinus carpio, (B) vampire characin, Hydrolycus scomberoides, (C) catfish Arius felis. (D) cod Gadus morhua. (E) large-mouth bass, Micropterus salmoides (F) The parrotfish Scarus guacamaia. Scale bar = 10 mm WESTNEAT 2004 From an evolutionary standpoint, fishes were the first animals to develop bony jaws. Versatile jaws and multiple feeding strategies allowed fishes to fill, or radiate into, a diverse range of niches. They have evolved to feed in all possible ways – sucking, biting, scraping, nipping, crushing etc. The head of a teleost has 5 main regions: Cranium, jaws, cheeks, hydroid arch, opercula. The head of a fish has five main regions • 1) The CRANIUM is composed of the bones providing direct support and protection to the brain and the visual, Anterior Posterior olfactory, and auditory organs. Below the cranium is the parashenoid bone. • Parasphenoid plays a role in the jaws as Features of the neurocranium sensu lato (from Caranx it acts as a hard melampygus, lateral aspect, left, and posterior aspect, right). A = prevomer, B = ethmoid, C = frontal, D = palate supraoccipital, E = pterotic, F = exoccipital, G = basioccipital, H = foramen magnum, I = parasphenoid, J = orbit. The five main regions Bowfin 2) The JAWS • Lower Jaw – has an Angular articular and dentary bone • Angular articular- The paired bones form the posterior part of either side of the lower jaw and articulate with the suspensorium.