NPS Discovery Toolkit » Metonitazene
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Etodesnitazene
Etodesnitazene Sample Type: Biological Fluid Latest Revision: February 23, 2021 Date of Report: February 23, 2021 1. GENERAL INFORMATION IUPAC Name: 2-[2-[(4-ethoxyphenyl)methyl]benzimidazol-1-yl]-N,N-diethyl- ethanamine InChI String: InChI=1S/C22H29N3O/c1-4-24(5-2)15-16-25-21-10-8-7-9-20(21)23- 22(25)17-18-11-13-19(14-12-18)26-6-3/h7-14H,4-6,15-17H2,1-3H3 CFR: Not Scheduled (02/2021) CAS# Not available Synonyms: Etazene, Desnitroetonitazene, Etazen, Etazone Source: Oregon State Police Forensic Laboratory Important Notes: All identifications were made based on evaluation of analytical data (LC-QTOF-MS) in comparison to analysis of acquired reference material. This drug was also confirmed via LC-MS/MS. Prepared By: Janet Schultz, PhD; Sailee Raje; Sara Short, MS, D-ABFT-FT; Michele Stauffenberg, MD; Alex J. Krotulski, PhD; Melissa F. Fogarty, MSFS, D-ABFT-FT; and Barry K. Logan, PhD, F-ABFT 2. CHEMICAL DATA Chemical Molecular Molecular Exact Mass Analyte Formula Weight Ion [M+] [M+H]+ Etodesnitazene C22H29N3O 351.5 351 352.2383 3. SAMPLE HISTORY Etodesnitazene has been identified in one case since December 2020. The geographical and demographical breakdown is below: Geographical Location: Oregon (n=1) Biological Sample: Subclavian Blood (n=1) Date of First Receipt: December 2020 Other Notable Findings: Etizolam, Methamphetamine, Mitragynine 4. BRIEF DESCRIPTION Etodesnitazene is classified as a novel opioid of the benzimidazole sub-class and is structurally dissimilar from fentanyl. Novel opioids have been reported to cause psychoactive effects similar to heroin, fentanyl, and other opioids. -

Communicationfile-132886.Pdf
From: Nazlee Maghsoudi To: Board of Health Cc: Werb, Daniel; Hayley Thompson; Karen McDonald Subject: BOH June 14, 2021 - CDPE Comments on HL29.2 Date: June 13, 2021 6:52:20 PM Attachments: 2021-06-14_CDPE Comments to BOH_Toronto Overdose Action Plan Status Report 2021.pdf To the Members of the Board of Health, Please find attached comments from the Centre on Drug Policy Evaluation (CDPE) regarding agenda item HL29.2 (Toronto Overdose Action Plan: Status Report 2021) to be considered at the Board of Health meeting on Monday, June 14. We would greatly appreciate if you could please confirm receipt. Thanks so much, Nazlee Maghsoudi, BComm, MGA Doctoral Candidate, Health Services Research | Institute of Health Policy, Management and Evaluation, University of Toronto Manager, Policy Impact Unit | Centre on Drug Policy Evaluation (CDPE) Chairperson, Executive Committee | New York NGO Committee on Drugs (NYNGOC) Strategic Advisor | Canadian Students for Sensible Drug Policy (CSSDP) (647) 702-7825 Pronouns: She/Her Submission from Nazlee Maghsoudi, Centre on Drug Policy Evaluation Toronto's Dru·g Checking Service Coordinated by the Centre on Drug Policy Evaluation > i" Comments for Board of Health Consideration of “Toronto Overdose Action Plan: Status Report 2021” June 14, 2021 What does Toronto’s drug checking service do? • Offers people who use drugs timely and detailed information on the contents of their drugs, helping them to make more informed decisions • Shares information on Toronto’s unregulated drug supply to help harm reduction workers and clinicians tailor the care they provide to people who use drugs • Advocates for services and safer alternatives for people who use drugs 2 Free and anonymous drug checking is now available! What you give .. -

WHO Expert Committee on Drug Dependence
WHO Technical Report Series 1034 This report presents the recommendations of the forty-third Expert Committee on Drug Dependence (ECDD). The ECDD is responsible for the assessment of psychoactive substances for possible scheduling under the International Drug Control Conventions. The ECDD reviews the therapeutic usefulness, the liability for abuse and dependence, and the public health and social harm of each substance. The ECDD advises the Director-General of WHO to reschedule or to amend the scheduling status of a substance. The Director-General will, as appropriate, communicate the recommendations to the Secretary-General of the United Nations, who will in turn communicate the advice to the Commission on Narcotic Drugs. This report summarizes the findings of the forty-third meeting at which the Committee reviewed 11 psychoactive substances: – 5-Methoxy-N,N-diallyltryptamine (5-MeO-DALT) WHO Expert Committee – 3-Fluorophenmetrazine (3-FPM) – 3-Methoxyphencyclidine (3-MeO-PCP) on Drug Dependence – Diphenidine – 2-Methoxydiphenidine (2-MeO-DIPHENIDINE) Forty-third report – Isotonitazene – MDMB-4en-PINACA – CUMYL-PEGACLONE – Flubromazolam – Clonazolam – Diclazepam The report also contains the critical review documents that informed recommendations made by the ECDD regarding international control of those substances. The World Health Organization was established in 1948 as a specialized agency of the United Nations serving as the directing and coordinating authority for international health matters and public health. One of WHO’s constitutional functions is to provide objective, reliable information and advice in the field of human health, a responsibility that it fulfils in part through its extensive programme of publications. The Organization seeks through its publications to support national health strategies and address the most pressing public health concerns of populations around the world. -

Benzimidazole-Opioids June 2021
Drug Enforcement Administration Diversion Control Division Drug & Chemical Evaluation Section Benzimidazole-Opioids June 2021 Introduction: metonitazene, metodesnitazene, and protonitazene involved interaction with β-arrestin-2. Mu-opioid receptor and β-arrestin-2 Recently, several synthetic substances of benzimidazole interaction has been implicated in adverse health effects of many structural class are being trafficked and abused for their opioid- opioid analgesics. It is well established that mu-opioid receptor like effects. In the late 1950s, the pharmaceutical research agonists have a high potential for addiction and can produce dose- laboratories of the Swiss chemical company CIBA dependent respiratory depression and arrest. Abuse of these Aktiengesellschaft synthesized numerous benzimidazole- benzimidazole-opioids has led to their positive identification in opioids to include butonitazene, etodesnitazene, flunitazene, several toxicological cases in the United States. Specifically, metonitazene, metodesnitazene, and protonitazene. Since metonitazene has been identified in twenty post-mortem cases. 2019, the abuse of benzimidazole-opioids as evidenced by their identification in toxicology cases, similar to other synthetic opioids, has resulted in adverse health effects including deaths. User Population: As the United States continues to experience an unprecedented The population likely to abuse benzimidazole-opioids appears to be epidemic of opioid misuse and abuse, the continued evolution the same as those abusing prescription opioid analgesics, heroin, and increased trafficking and popularity of new and deadly and other synthetic opioid substances. This is evidenced by the synthetic opioids including benzimidazole-opioids with no types of other drugs co-identified in isotonitazene seizures and in approved medical use are of public health concern. fatal overdose cases. Toxicology analyses co-identified some of these benzimidazole-opioids with other opioids, stimulants, and Chemistry: benzodiazepines. -

Metonitazene Begins Proliferation As Newest Synthetic Opioid Among Latest Cycle of Non-Fentanyl Related Drugs
January 2021 Metonitazene Begins Proliferation as Newest Synthetic Opioid Among Latest Cycle of Non-Fentanyl Related Drugs Purpose: The objective of this announcement is to notify public health and safety, law enforcement, first responders, clinicians, medical examiners and coroners, forensic and clinical laboratory personnel, and all other related Demographics communities about new information surrounding the emergent synthetic opioid metonitazene. Case Type: • Postmortem (n=8) Background: Synthetic opioids are chemically manufactured drugs, often accompanied with unknown potency and adverse effects or health risks. New synthetic opioids may be mixed with more traditional opioids, creating additional Age: • Range: 30s to 50s risk and danger for recreational drug users. Synthetic opioids may be distributed in powder or tablet form. In the United States (U.S.), an alarming increase in the number of deaths linked to synthetic opioid use has been reported. Date of Collection: • Aug. to Dec. 2020 The primary adverse effect associated with synthetic opioid use is respiratory depression, often leading to death. Other Notable Findings: Summary: Metonitazene is a potent synthetic opioid bearing structural resemblance to etonitazene, a synthetic opioid • Fentanyl (n=6) that is nationally and internationally controlled. Metonitazene is dissimilar in structure to other synthetic opioids • Cocaine (n=4) typically encountered in forensic casework (e.g. fentanyl analogues). Metonitazene and similar analogues (e.g. • Methamphetamine (n=4) etonitazene, isotonitazene) were first synthesized and reported in the literature in the 1950s. Pharmacological data suggest that this group of synthetic opioids have potency similar to or greater than fentanyl. Metonitazene was first reported by NPS Discovery after detection in a seized drug powder in July 2020. -

NFLIS-Drug Selected Substance List
2017-2020 NFLIS-Drug Substance List (Sorted by Date) Date Added NFLIS Substance Name Synonyms Chemical Name Structure InChI Formula to NFLIS- Drug InChI=1S/C16H20BrN/ c17-14-1-3-15(4-2-14)18-16-12-6-10-5-11 Bromantane ladasten N-(4-bromophenyl)adamantan-2-amine C16H20BrN 12/7/20 (8-12)9-13(16)7-10/h1-4,10-13,16,18H, 5-9H2 InChI=1S/C21H29FN2O3/ c1-4-27-21(26)19(15(2)3)23-20(25)17-14- ethyl 2-(1-(5-fluoropentyl)-1H-indole-3-carboxamido)-3- 5F-EMB-PICA EMB-2201; 5-fluoro-EMB-PICA 24(13-9-5-8-12-22)18-11-7-6-10-16(17)18 C21H29FN2O3 11/12/20 methylbutanoate /h6-7,10-11,14-15,19H, 4-5,8-9,12-13H2,1-3H3,(H,23,25) InChI=1S/C20H27FN2O3/ c1-20(2,3)17(19(25)26-4)22-18(24)15-13- methyl 2-(1-(4-fluorobutyl)-1H-indole-3- 4F-MDMB-BUTICA 4-fluoro-MDMB-BUTICA; 4F-MDMB-BICA 23(12-8-7-11-21)16-10-6-5-9-14(15)16/ C20H27FN2O3 10/23/20 carboxamido)-3,3-dimethylbutanoate h5-6,9-10,13,17H,7-8,11-12H2,1-4H3,(H, 22,24) InChI=1S/C10H14BrNO2/ 4-methoxy-6-[(1E)-2-phenylethenyl]-5,6-dihydro-2H- 2Br-4,5-Dimethoxyphenethylamine 2-bromo-4,5-dimethoxyphenethylamine c1-13-9-5-7(3-4-12)8(11)6-10(9)14-2/ C10H14BrNO2 10/2/20 pyran-2-one h5-6H,3-4,12H2,1-2H3 InChI=1S/C16H22FNO/ 4-fluoro-3-methyl-alpha-PVP; 4F-3-methyl-alpha- c1-3-6-15(18-9-4-5-10-18)16(19)13-7-8-1 4F-3-Methyl-alpha-PVP 4-fluoro-3-methyl-alpha-pyrrolidinopentiophenone C16H22FNO 10/2/20 pyrrolidinovalerophenone 4(17)12(2)11-13/h7-8,11,15H, 3-6,9-10H2,1-2H3 InChI=1S/C21H26N4O3/ N,N-diethyl-2-[2-(4-methoxybenzyl)-5-nitro-1H- c1-4-23(5-2)12-13-24-20-11-8-17(25(26)2 Metonitazene C21H26N4O3 9/15/20 benzimidazol-1-yl]ethanamine -
![ADVANCED RELEASE EMCDDA Technical Report on the New Psychoactive Substance N,N- Diethyl-2-[[4-(1-Methylethoxy)Phenyl]Methyl]-5-N](https://docslib.b-cdn.net/cover/1311/advanced-release-emcdda-technical-report-on-the-new-psychoactive-substance-n-n-diethyl-2-4-1-methylethoxy-phenyl-methyl-5-n-2031311.webp)
ADVANCED RELEASE EMCDDA Technical Report on the New Psychoactive Substance N,N- Diethyl-2-[[4-(1-Methylethoxy)Phenyl]Methyl]-5-N
ADVANCED RELEASE EMCDDA technical report on the new psychoactive substance N,N- diethyl-2-[[4-(1-methylethoxy)phenyl]methyl]-5-nitro-1H- benzimidazole-1-ethanamine (isotonitazene) Explanatory note: In the interests of public health protection the EMCDDA is releasing this report before formal copy editing and page layout in the EMCDDA house style. The final report will be available on the EMCDDA website in due course. Authors: Michael Evans-Brown1, István Ujváry2, Joanna De Morais1, Rachel Christie1, Anabela Almeida1, Rita Jorge1, Ana Gallegos1, Roumen Sedefov1 1European Monitoring Centre for Drugs and Drug Addiction, Praça Europa 1, Cais do Sodré, 1249–289 Lisbon, Portugal 2iKem BT, Búza u. 32, Budapest 1033, Hungary Recommended citation: EMCDDA (2020), EMCDDA technical report on the new psychoactive substance N,N-diethyl-2-[[4-(1-methylethoxy)phenyl]methyl]-5-nitro-1H- benzimidazole-1-ethanamine (isotonitazene), EMCDDA, Lisbon. © European Monitoring Centre for Drugs and Drug Addiction, 2019 Praça Europa 1, Cais do Sodré, 1249–289 Lisbon, Portugal Tel: +351 211210200 Email: [email protected] Web: www.emcdda.europa.eu 1 Purpose The purpose of this technical report to provide an analyses of the available information on N,N-diethyl-2-[[4-(1-methylethoxy)phenyl]methyl]-5-nitro-1H-benzimidazole-1-ethanamine (commonly known as isotonitazene), an opioid analgesic that has recently emerged on the drug market in Europe, to support a risk assessment of the substance that has been requested by the European Commission in accordance with Article 5c of Regulation (EC) No 1920/2006 (as amended). Parts of this report were prepared under an EMCDDA contract (ref. -

Synthetic Opioids
Clinical Update: July 2021 NPS FOCUS: “NITAZENE” SYNTHETIC OPIOIDS Aegis launched an extensive and industry-leading testing menu of Novel Psychoactive Substances (NPS) in mid- 2020, which includes phenibut, tianeptine, xylazine, designer benzodiazepines, designer opioids, hallucinogens/dissociatives, synthetic cannabinoids, and synthetic stimulants, and continues to expand. In this month’s update, we will focus on the evolution of the non-fentanyl opioid analogue drug class commonly referred to as “nitazenes” and the challenges associated with detection and monitoring of these illicit substances. Origins of the “nitazenes” have been traced back to decades old pharmaceutical industry attempts to develop a novel opiate alternative to morphine.1-2 However, due to a high risk for abuse potential and reports of an overdose in early human studies, the “nitazenes” were deemed “not suitable for human consumption” and never gained approval for clinical use. With the increased regulation of older substances such as fentanyl and Schedule 1 illegal drugs, more varied chemical classes of opioids are in high demand. In response, the “nitazenes” have recently been “re-discovered” as an equally effective substitute and are illicitly marketed as a drug less detected in drug monitoring programs. Isotonitazene (also referred to as “iso”), is the prototypical, most frequently encountered member of the “nitazenes”. Iso is an extremely potent mu opioid receptor agonist and is structurally unrelated to fentanyl or traditional opioids.1 It first appeared in Canada and Europe in March 2019. By July 2019, isotonitazene was found in biological samples in the United States and has since been identified in over 250 drug overdose deaths. -
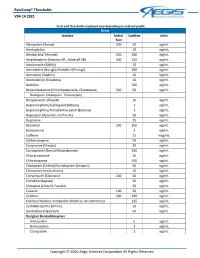
Paincomp® Thresholds V04.14.2021 Copyright © 2020 Aegis Sciences
PainComp® Thresholds V04.14.2021 Tests and thresholds employed vary depending on ordered profile Urine Analyte Initial Confirm Units Test Alprazolam (Xanax) 200 50 ng/mL Amitriptyline 10 ng/mL Amobarbital (Amytal) 200 200 ng/mL Amphetamine (Adzenys ER , Adderall XR) 500 250 ng/mL Aripiprazole (Abilify) 10 ng/mL Armodafinil (Nuvigil)/Modafinil (Provigil) 100 ng/mL Asenapine (Saphris) 10 ng/mL Atomoxetine (Strattera) 10 ng/mL Baclofen 500 ng/mL Benzodiazepines (Chlordiazepoxide, Clorazepate, 200 50 ng/mL Diazepam, Oxazepam, Temazepam) Brexpiprazole (Rexulti) 10 ng/mL Buprenorphine/Sublingual (Belbuca) 1 ng/mL Buprenorphine/Transdermal patch (Butrans) 1 ng/mL Bupropion (Aplenzin, Forfivo XL) 50 ng/mL Buspirone 25 ng/mL Butalbital 200 200 ng/mL Butorphanol 1 ng/mL Caffeine 15 mcg/mL Carbamazepine 50 ng/mL Cariprazine (Vraylar) 20 ng/mL Carisoprodol (Soma)/Meprobamate 200 ng/mL Chlorpromazine 10 ng/mL Chlorzoxazone 500 ng/mL Citalopram (Celexa)/Escitalopram (Lexapro) 50 ng/mL Clomipramine (Anafranil) 10 ng/mL Clonazepam (Klonopin) 200 50 ng/mL Clonidine (Kapvay) 50 ng/mL Clozapine (Clozaril, Fazaclo) 50 ng/mL Cocaine 100 50 ng/mL Codeine 200 100 ng/mL Cotinine/Nicotine metabolite (Habitrol, Nicoderm CQ) 125 ng/mL Cyclobenzaprine (Amrix) 10 ng/mL Dantrolene (Dantrium) 50 ng/mL Designer Benzodiazepines Adinazolam 5 ng/mL Bromazolam 1 ng/mL Clonazolam 1 ng/mL Copyright © 2020 Aegis Sciences Corporation All Rights Reserved PainComp® Thresholds V04.14.2021 Deschloroetizolam 1 ng/mL Diclazepam 1 ng/mL Etizolam 1 ng/mL Flualprazolam 1 ng/mL Flubromazepam -

2020 Drug Related Death Report
2020 DRUG-RELATED DEATH REPORT Knox and Anderson County, Tennessee Dr. Darinka Mileusnic-Polchan Chief Medical Examiner Knox & Anderson County Chris Thomas Chief Administrative Officer Knox County Regional Forensic Center WWW.KNOXCOUNTY.ORG/RFC/ 2020 Drug-Related Death Report Table of Contents Letter from the Chief Medical Examiner...................................................................................................................... 2 2020 Key Findings ...................................................................................................................................................... 4 Knox & Anderson Counties Age Distribution for Drug-Related Deaths 2010-2020 ......................................................... 5 Knox & Anderson Counties Gender Distribution for Drug-Related Deaths 2010-2020 ................................................. 11 Knox County Race Distribution for Drug-Related Deaths 2010-2020........................................................................... 16 Knox & Anderson Counties 2020 Drug-Related Deaths by Manner of Death ............................................................... 18 Knox & Anderson Counties 2020 Drug-Related Deaths by Location of Occurrence ...................................................... 19 Knox & Anderson Counties Zip Code Distribution and Heat Maps 2020 ...................................................................... 21 Knox County Heat Maps Home, Injury, and Death Locations ........................................................................ -

Isotonitazene Initial Report
INITIAL REPORTS 11 ISSN 0000-0000 2600-0954 UtminusamasfsitibusaIsotonitazene EMCDDAvolupta initial report EMCDDA on the new psychoactive aut substance N,N-diethyl-2-[[4-(1-methylethoxy)phenyl] Ernam sequiatet omnis sundige neceprem cus methyl]-5-nitro-1H-benzimidazole-1-ethanamine dic te dolum est, custiat asperis quia sectae (isotonitazene) consequis dem landandae eveliqu iatusap isciunti officiis corest, comniet ommodio In accordance with Article 5b of Regulation (EC) No 1920/2006 (as amended) About this publicationseries NisitatEMCDDA quationse initial reports numque are voluptas drawn up assunt on one or several similar new quam,psychoactive eat aliquos substances nis ant fuga.that may Ur alit pose health or social risks at European quiUnion veliqui level. netur? Iquas ipsusa verchil Initialmagnimusam, reports provide tem fugias scientific atemodi evidence num lit to the Commission in order to allow itvera to makeuis nonsequiam, an informed siminus decision mi, regarding nonem whether or not there is a need to requesteaqui tende a risk voluptate assessment num. on a new psychoactive substance. INITIAL REPORT I Isotonitazene Contents Acknowledgements ................................................................................................................. 3 Statement regarding the United Kingdom ............................................................................... 4 1. Introduction .......................................................................................................................... 5 2. Information -

Softember Abstracts
SOFTEMBER ABSTRACTS SOFTEMBER POSTER ABSTRACTS...............................................2-56 SOFTEMBER PLATFORM ABSTRACTS .................................... 57-127 SOFTEMBER POSTER ABSTRACTS A 4-Year Systematic Analysis of Commercial E-liquids: The Evolution of G.R.A.S. to Inhaled Toxins Authors and Affiliations: Alaina K. Holt, B.S.*(1), Justin L. Poklis, B.S.(2), and Michelle R. Peace, Ph.D.(1) (1) Department of Forensic Science and (2) Department of Pharmacology & Toxicology, Virginia Commonwealth University, Richmond, VA Background/Introduction: Electronic cigarettes (e-cigs) were originally developed as an alternate method for nicotine delivery. The e-liquids are made of ratios of humectant formulations, pharmacologically active ingredients, flavoring chemicals, volatiles, preservative chemicals, and other “enhancing” chemicals. E-cigs have also been adopted for drugs other than nicotine (DOTN), including cannabidiol (CBD) and tetrahy- drocannabinol (THC). E-liquid manufacturers use chemicals that are generally regarded as safe (GRAS) for oral consumption. The FDA promulgated regulations to govern e-cigarette devices and e-liquids in May 2016, yet product approval deadlines are May 2020. The flavoring bans that were instituted in January 2020 only govern pod-based products. E-liquid formulations for re-filling e-cigarettes have not been heavily scrutinized by regulatory agencies, under the auspice that only pod-based products are most likely to be used by children. E-liquid formulations have evolved as a result of vaping preferences, public health sentiment, and looming regulations. Quality assurance and product safety remain inadequate, demonstrated recently by e-cigarette or vaping product associated lung injury (EVALI). Objectives: The objective of this study was to evaluate the chemical composition of e-liquids and to establish over time humectant formulations, pharmacologically active ingredients, flavoring chemicals, volatiles, preservative chemicals, and other “enhancements”.