Press Release
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Email Ids of Advocate General, Government Pleaders, Public
LIST OF SPL. GOVT. PLEADERS, GOVT. PLEADERS All the learned advocates are requested to serve the copies of petition and material papers to the learned Advocate General or Government Pleaders(when shown as Respondent) through email as shown in the given table. The learned Advocates also requested to mention the said fact about the service in the filing process(in email). Name of the Spl.GP/GP Sl.No. Department Mobile Number Mail Id Remarks S/Sri 1 B.Shivananda Prasad Advocate General [email protected] 3 HarenderPershad, Spl. GP Attached to the A.G. 9866325458 [email protected] 4 P.Radhive, (GP) Attached to the A.G. 9391647666 [email protected] AdullapallySanthosh Kumar, 5 Attached to the AG 9394000069 [email protected] (GP) 6 S.Sharath Kumar, Spl. GP Attached to the Addl.A.G. 7032704731, 9849444972 [email protected] AndapalliSanjeev Kumar, Spl. 7 Attached to the Addl.A.G. 7032704733, 9848332626 [email protected] GP 8 SripathiSanthosh Kumar, (GP) Attached to the Addl.A.G. 9949195001 [email protected] Roads and Building, Youth 9 B.Jayakar, (GP) Advancement, Tourism, Culture 9849133705 [email protected] and Archaeology Dept General Administration, Information Technology, Electronics & 10 PalleNageswaraRao, (GP) Communications, Finance and Planning 9848022187 [email protected] Depts. Prohibition & Excise, Labour, 11 G.Arun Kumar, (GP) Employment, Training & Factories 9440544613 [email protected] Dept Health, Medical & Family [email protected] 12 NageshBheemapaka Welfare Department. 9848012711 Panchayat Raj & Rural 13 Smt.G.JyothiKiran, (GP) Development, Consumer Affairs, 9177696938 [email protected] Food & Civil Supplies Depts Industries, Mines & Commerce, Infrastructure & 14 T.V.RamanaRao, (GP) 9849012070 [email protected] Investment, Pubilic Enterprises Dept Irrigation and Command [email protected] 15 N.V.Shravan Kumar, (GP) 9866774739, 9949828131 Area Dev. -

Review of Research
Review of ReseaRch HISTORY OF REDDY KINGDOM Mallanagouda S.Hadalageri and Dr.Suresh N Hullannavar issN: 2249-894X impact factoR : 5.7631(Uif) UGc appRoved JoURNal No. 48514 volUme - 8 | issUe - 8 | may - 2019 ABSTRACT: "Reddy likewise transliterated as Raddi, Reddi, Reddiar, Reddappa, Reddy and so on is a position begun in India, dominatingly settled in Andra Pradesh, Telangana, Karnataka, Kerala, Tamil Nadu, Pandicheri. They are named a forward station. The inception of the Reddy has been connected to the Rashtrakutas, despite the fact that sentiments change. At one time they were a warrior station and later become medieval overlords and worker owners. [1][2] Historically they have been the land-possessing nobility of the towns. [3][4][5]Traditionally, they were a different network of shippers and cultivators. [1][6][7] Their ability as rulers and warriors is all around archived in Telagu history. [8] The Reddy line (1325-1448 CE) administered beach front and focal Andra for over a hundred years." KEYWORDS: Reddy, Dynasty, holding community held much kingdom conquered by the Rastrakuta, Vijaynagar, respects next to Brahmins. chalukkiars about the fourth Kakatiya, British, Political According to Rev. J Foulks the century A.D. J.F. fleet writes that Participation. Reddys are called under various Reddy’s first appearance found names, Rattu, Ruth, Rattu, Reddi at Kanarese of Bombay in the II INTRODUCTION: etc. Dr. Burmell points out that the fifth century A.D. They were Reddys are the major thriving family of Reddis belonged to interrupted with the attack of communities of India Dravidian origin and states the Pallavas and other rulers significant since medieval Rashtraas an instance of during the eighth century A.D. -

Vamshi Krishna Reddy
Dr. V. Vamshi Krishna Reddy Assistant Professor, Centre for Comparative Literature, School of Humanities, University of Hyderabad, Hyderabad - 500046 | Mobile: +918280468530 | Mail: [email protected], [email protected] Education · Ph.D. (2014) in Comparative Literature from University of Hyderabad PhD Dissertation Title: “Cinema and Politics in Andhra Pradesh” · M.Phil. (2008) in Comparative Literature from University of Hyderabad M.Phil. Dissertation Title: “Ram Gopal Varma’s Films: A Selected Study” · M.A. (2006) in English from University of Hyderabad · B.A. English Literature (2004) from Nizam College, Hyderabad Specialization & Areas of Interest · Cultural Studies, Film Studies and Critical Theory Teaching Experience · 2020 – Present Centre for Comparative Literature, School of Humanities, University of Hyderabad, Hyderabad as an Assistant Professor in Comparative Literature · 2017 – 2020: Department of Humanities and Social Sciences, Indian Institute of Technology, Tirupati, Andhra Pradesh as an Assistant Professor in English · 2014 - 2017: Department of Humanities and Social Sciences, National Institute of Technology, Rourkela, Odisha as an Assistant Professor in English Ongoing Sponsored Research Projects No. Project Title Funding Agency Funding Value 1 Folk Media and Identity Construction: Production and IMPRESS, 7,00,000/- Dissemination in Second Phase Telangana Movement ICSSR Seminars and Conferences · Presented a paper titled “Channels of Discourse: You Tube and The Emerging Regional Digital ‘Media Culture’ -

Tank, Temple and Town Policy - Construction of Water Tanks (Water Conservation Structures) Resulting in Prosperity of Towns
Component-I (A) – Personal details: Prof. P. Bhaskar Reddy Sri Venkateswara University, Tirupati. Dr. Ravi Korisettar, UGC Emeritus Fellow Karnatak University, Dharwad. Onkar Tendulkar Virasat E Hind Foundation. Bombay. Dr. Ravi Korisettar Karnatak University, Dharwad. 1 Component-I (B) – Description of module: Subject Name Indian Culture Paper Name Art and Architecture of India Module Name/Title Art and Architecture under Kakatiya dynasty Module Id IC / AAI / 01 Pre requisites Understanding the evolution and salient features of Objectives Kakatiya Dynasty art and architecture Dravida School of temple architecture, Warangal, Keywords Ramappa temple, Hanamkonda, Telangana E-text (Quadrant-I) : 1. Introduction Kakatiyas- an indigenous Telugu dynasty ruled over the Andhradesa consisting of modern states of Telangana and Andhra Pradesh from 10th century AD to the first quarter of 14th century AD. The name ‘Kakatiya’ is derived from goddess Kakati- a mother goddess Durga, venerated by the dynasty. Gunadya Rashtrakuta was the first known personality of the Kakatiya clan. He was the commander of the Rashtrakuta Krishna II who died in the battlefield while fighting with the Eastern Chalukyas. King Krishna II, very pleased with Gundaya’s loyal service towards him, rewarded Ereya, Gunadya’s son with the governorship of the Korivi region. Kakatiyas were the feudatories of Rashtrakuta kings upto 10th century AD after which they were overthrown by the Kalyana Chalukyas. Kakatiyas then became the feudatories or samanthas of Kalyana Chalukyans. The Kakatiya rulers Beta I (AD 996–1051), Prola I (AD 1052–1076), Beta II (AD 1076–1108), Durgaraja (AD 1108–1116) and Prola II (AD 1116– 1157) served the Kalyana Chalukyas until the collapse of Chalukya empire during Tailapa III’s reign. -
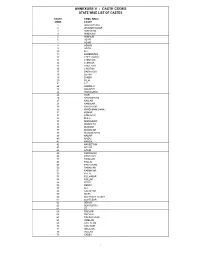
Annexure V - Caste Codes State Wise List of Castes
ANNEXURE V - CASTE CODES STATE WISE LIST OF CASTES STATE TAMIL NADU CODE CASTE 1 ADDI DIRVISA 2 AKAMOW DOOR 3 AMBACAM 4 AMBALAM 5 AMBALM 6 ASARI 7 ASARI 8 ASOOY 9 ASRAI 10 B.C. 11 BARBER/NAI 12 CHEETAMDR 13 CHELTIAN 14 CHETIAR 15 CHETTIAR 16 CRISTAN 17 DADA ACHI 18 DEYAR 19 DHOBY 20 DILAI 21 F.C. 22 GOMOLU 23 GOUNDEL 24 HARIAGENS 25 IYAR 26 KADAMBRAM 27 KALLAR 28 KAMALAR 29 KANDYADR 30 KIRISHMAM VAHAJ 31 KONAR 32 KONAVAR 33 M.B.C. 34 MANIGAICR 35 MOOPPAR 36 MUDDIM 37 MUNALIAR 38 MUSLIM/SAYD 39 NADAR 40 NAIDU 41 NANDA 42 NAVEETHM 43 NAYAR 44 OTHEI 45 PADAIACHI 46 PADAYCHI 47 PAINGAM 48 PALLAI 49 PANTARAM 50 PARAIYAR 51 PARMYIAR 52 PILLAI 53 PILLAIMOR 54 POLLAR 55 PR/SC 56 REDDY 57 S.C. 58 SACHIYAR 59 SC/PL 60 SCHEDULE CASTE 61 SCHTLEAR 62 SERVA 63 SOWRSTRA 64 ST 65 THEVAR 66 THEVAR 67 TSHIMA MIAR 68 UMBLAR 69 VALLALAM 70 VAN NAIR 71 VELALAR 72 VELLAR 73 YADEV 1 STATE WISE LIST OF CASTES STATE MADHYA PRADESH CODE CASTE 1 ADIWARI 2 AHIR 3 ANJARI 4 BABA 5 BADAI (KHATI, CARPENTER) 6 BAMAM 7 BANGALI 8 BANIA 9 BANJARA 10 BANJI 11 BASADE 12 BASOD 13 BHAINA 14 BHARUD 15 BHIL 16 BHUNJWA 17 BRAHMIN 18 CHAMAN 19 CHAWHAN 20 CHIPA 21 DARJI (TAILOR) 22 DHANVAR 23 DHIMER 24 DHOBI 25 DHOBI (WASHERMAN) 26 GADA 27 GADARIA 28 GAHATRA 29 GARA 30 GOAD 31 GUJAR 32 GUPTA 33 GUVATI 34 HARJAN 35 JAIN 36 JAISWAL 37 JASODI 38 JHHIMMER 39 JULAHA 40 KACHHI 41 KAHAR 42 KAHI 43 KALAR 44 KALI 45 KALRA 46 KANOJIA 47 KATNATAM 48 KEWAMKAT 49 KEWET 50 KOL 51 KSHTRIYA 52 KUMBHI 53 KUMHAR (POTTER) 54 KUMRAWAT 55 KUNVAL 56 KURMA 57 KURMI 58 KUSHWAHA 59 LODHI 60 LULAR 61 MAJHE -

Prof. J. Mahender Reddy Vice-Chancellor IFHE, Hyderabad
Prof. J. Mahender Reddy Vice-Chancellor IFHE, Hyderabad Prof. J Mahender Reddy is at present Vice-Chancellor of ICFAI Foundation for Higher Education, a Deemed-to-be University under section 3 of the UGC Act, located in Hyderabad. As the Vice-Chancellor he has significantly contributed to the development of a research culture and a good leadership which enabled the University to secure ‘A’ grade by the National Assessment and Accreditation Council (NAAC). He is the Chancellor of ICFAI University Tripura and was the Chancellor of ICFAI University Raipur. Prof. Reddy got his Master’s degree from The Delhi School of Economics with distinction and a doctorate in Economics from the University of Alberta in Edmonton, Canada. He was a Fellow of the Shastri Indo-Canadian Institute, at Carleton University in Ottawa, Canada in, and again at the University of Ottawa. He was Fulbright Fellow, at the University of Boston. He was the first Chair, in the Indian Ocean Studies, of the Indian Ocean Rim Association for Regional Cooperation, located at the University of Mauritius. He taught in the Faculty of Business Administration, at the University of Alberta in Canada, Saint Peter’s College in the United States, Administrative staff College of India, and at the Osmania University for over three decades, and won the Best Teacher award from the Government of Andhra Pradesh. He has guided 14 PhDs and 9 M.Phil scholars in Osmania and ICFAI Universities. He has published forty research papers, and ten books. He was a member of the High Level Committees appointed by the UGC, ICSSR, the Government of Andhra Pradesh, and the government of India, Association of Management Development Institutions in South Asia (AMDISA), and ICFAI. -

Rank Obtained Sl
Provisional Integrated inter -se seniority list of Grade -I Village Revenue Officers in respect of Y.S.R. District Rank obtained Sl. Name of the Mandai in Name of the Village Date of Joining Educational Name of the Caste If Physically by theVROs No. Name of the Gr-I VRO Date of birth Handicapped which VRO working Secretariat as VRO Qualification caste category (Yes/No) recruitedthrough APPSC 1 2 3 4 5 6 7 8 9 10 11 1 Gorentia Balaiah CllaPaao- Madttru -0_8-02-2007 01-09~1961 SSC Sale BCB No - 2 - Kakanuru Chandra Sekhar Reddy Proddatur Dorasanaipalli 08-02-2007 15-11-1961 SSC - - Kapu OC __ .~No - 3 C,V Satyanarayana Rao - Simhadripuram Kovaramguttapalli 08-02-2007 10-06-1962 sse Brahmin oe No - 4 S,Ramakrishna Reddy Porumamilia Rangasamudram 08-02-2007 24-08-1962 Intermediate Kapu OC No - 5 Pasam Prasad Lakkireddipalii Maddirevula 08-02-2007 05-04-1963 sse Mala SC No - 6 B,V.Subba Reddy Chapadu Chinnaguravalur 08-02-2007 01-07-1963 Intermediate Kapu OC No - 7 N.Suresh Lakshmi Babu Chennur Ramanapalli 08-02-2007 10-07-1963 sse Brahmin OC No - 8 S,Ramesh Reddy Chitvel Nethivaripalli 08-02-2007 21-07-1963 Intermediate Kapu OC No - 9 S,V. Narasimham Rajupalem Thondaladinne 08-02-2007 09-10-1963 Degree Brahmin OC No - 10 p, Dhanamma Obulavaripalli Obulavaripalli 08-02-2007 14-06-1964 sse Kamma OC No - 11 B,V.Subba Reddy CKDinne Thadigotla 08-02-2007 14-07-1964 Intermediate Kapu OC No - 12 V,Sai Prasada Rao Proddutur Somulavaripalli1 08-02-2007 01-03-1965 Intermediate Brahmin OC No 13 Mannuru Rama Subbareddy Penagalur Penagalur 08-02-2007 17-03-1965 Degree Kapu OC No - 14 Lroban Chitvel Nagavaram-1 08-02-2007 01-06-1966 Intermediate Mala SC No - 15 M.Pratap Reddy CKDinne Kopparthy 08-02-2007 20-05-1967 Intermediate Kapu OC No - 16 M Chandra Sekar Raju Nandalur Nagireddypalli-II 08-02-2007 01-06-1968 sse Kshatriya OC No - 17 P Venkata Sivamma Chapadu Alladupalli 08-02-2007 05-01-1969 SSC Yadava BCD No - 18 A. -

Natural Selection Intensity in Settibalija, a Mendelian Human Population from South India
Antrocom Online Journal of Anthropology 2011, vol. 7. n. 1 Prakash & Sudhakar – Natural Selection Intensity Physical Anthropology 111 – 115 Natural Selection Intensity in Settibalija, A Mendelian Human Population from South India Deva S.R.S. Prakash1 & Godi Sudhakar Department of Human Genetics, Andhra University, Visakhapatnam – 530 003, Andhra Pradesh, India Abstract The selection intensity indices were computed based on the demographic information pertaining to fertility and mortality among Settibalija, an endogamous Mendelian population of Andhra Pradesh, South India. The total fertility and mortality indices are slightly lower than other Andhra populations studied earlier. In the present caste population, the selection is manifested primarily through differential fertility rather than mortality, which is a not deviation from general trend. The results are discussed in the light of earlier studies on some caste and tribal populations inhabiting Andhra Pradesh, South India. Key words: Natural Selection; Fertility; Mortality; Andhra Caste. Introduction The concept of natural selection is well recognized as a principal driving force of evolution. Natural selection operates through differential mortality and fertility among human populations. The differential mortality acts on individuals prior to their reproductive age, and determines that group of individuals who survive and may potentially produce the offspring who will constitute the next generation of a population. Darwin’s concept of natural selection for the descent of the organisms which refers to the evolution, by which operates “survival of the fittest” referring differential mortality of individuals within a species and include the selection agent of differential fertility. It is probable that natural selection operating through differential mortality is less important among modern human populations where differential fertility appears to be the more effective agent. -

GOVERNMENT of TELANGANA ABSTRACT PUBLIC SERVICES – Postings of Certain Special Grade Deputy Collectors and Deputy Collectors
GOVERNMENT OF TELANGANA ABSTRACT PUBLIC SERVICES – Postings of certain Special Grade Deputy Collectors and Deputy Collectors - Orders – Issued. =-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=- GENERAL ADMINISTRATION (SPL.A) DEPARTMENT G.O.Rt.No. 2242 Dated: 11.10.2016, Read the following:- 1. G.O.Ms.No. 221 to 250 Revenue (DA-CMRF) Department, dt.11.10.2016.. 2. G.O.Rt.No. 2235, General Administration (Spl.A) Department, dt. 11.10.2016. 3. G.O.Rt.No.2236, General Administration (Spl.A) Department, dt. 10.10.2016. 4. G.O.Rt.No. 2239, General Administration (Spl.A) Department, dt. 11.10.2016. 4. G.O.Rt.No. 2241, General Administration (Spl.A) Department, dt. 11.10.2016. ***** ORDER: The following transfers and postings are ordered with immediate effect: 1. Sri J. Sudhakar Rao, Special Grade Deputy Collector is transferred and posted to Officiate in his own rank and pay as Joint Collector & Additional District Magistrate (Non-Cadre), Mancherial District. 2. Sri Ch. Shiva Lingaiah, Deputy Collector is transferred and posted to Officiate in his own rank and pay as Joint Collector & Additional District Magistrate (Non-Cadre), Nirmal District. 3. Sri V. Ashok Kumar, Special Grade Deputy Collector is transferred and posted to Officiate in his own rank and pay as Joint Collector & Additional District Magistrate (Non-Cadre), Komarambheem District, Headquarters at Asifabad. 4. Sri A. Nagendra, Special Grade Deputy Collector is transferred and posted to Officiate in his own rank and pay as Joint Collector & Additional District Magistrate (Non-Cadre), Jagityal District. 5. Sri S. Prabhakar Reddy, Special Grade Deputy Collector is transferred and posted to Officiate in his own rank and pay as Joint Collector & Additional District Magistrate (Non-Cadre), Peddapalli District. -

Gayatri Reddy CV
GAYATRI REDDY Office Address: Gender and Women’s Studies Program and Department of Anthropology 1820 University Hall (m/c 360), UIC 601, S. Morgan Street Chicago, IL 60607 Phone: 312-413-5658; Fax: 312-355-4478 EDUCATION 1992-2000 Emory University, Atlanta, GA Ph.D. Anthropology 1998-2000 Emory University, Atlanta, GA M.P.H, International Health (Community Health) 1991-1992 Columbia University, New York, NY M.A, Anthropology 1987-1990 University of Delhi, Delhi, India B.A, Psychology GRANTS AND FELLOWSHIPS 2005-2006 Institute for the Humanities Faculty Fellowship, University of Illinois at Chicago, Chicago, IL Fall, 2005 OVCR-AAH Grant, University of Illinois at Chicago, Chicago, IL Fall, 2004 Joan Heller-Diane Bernard Fellowship, Center for Lesbian and Gay Studies Center, CUNY, New York City, NY Spring, 2004 OVCR Seed Grant, Office of Social Science Research, UIC Fall, 2003 OVCR Micro Grant, Office of Social Science Research, UIC 2000-2002 Social Science Research Council Sexuality Postdoctoral Fellowship 1999-2000 Emory University Dean’s Teaching Fellowship 1998-1999 Mellon Foundation and Center for the Study of Health, Culture, Society Fellowship Rollins School of Public Health, Emory University 1995-1997 National Science Foundation Doctoral Research Grant 1995 Association for Women in Science (AWIS) Research Grant 1992-1998 Emory University Graduate Fellowship 1991-1992 Columbia University-Ford Foundation Faculty Fellowship PUBLICATIONS "Bonds of Love: The Desire for Companionate Marriages Among Hijras of India" to appear in edited volume Modern Love: Companionate Marriage and the Politics of Love, edited by Hirsch, J and H. Wardlow, University of Michigan Press, 2006. “Sexual Difference and its Discontents: Shifting Contexts of ‘Thirdness’ in Hyderabad, India (tentative title) to appear in edited volume Alternative Sexualities in India, edited by Brinda Bose, Seagull Press, 2006. -

Rani Rudrama Devi.Pdf
ISBN 978-81-237-7817-4 First Edition 2016 (Saka 1937) © Alekhya Punjala 2016 Rani Rudrama Devi (English) ` 145.00 Published by the Director National Book Trust, India Nehru Bhawan 5 Institutional Area, Phase-II Vasant Kunj, New Delhi - 110070 Website: www.nbtindia.gov.in CONTENTS Preface vii 1. History of the Kakatiyas 1 2. Reign of Rudrama Devi 15 3. Rani Rudrama Devi’s Persona 51 4. Patronage of Literature, Art, Architecture and Culture 55 Annexures Annexure I: Some Important Inscriptions from the Kakatiya Period 101 Annexure II: Chronology of the Kakatiya Dynasty 107 Bibliography 109 PREFACE Merely a couple of days ago, while going through the nomination process for Presidency, Hillary Clinton made a telling comment that she really didn’t know whether America was ready for a woman President. It is a matter of great pride that India has shown itself to be a path-breaker on this count. In mythology as well as since historical times we have worshipped goddesses and attributed the most important portfolios to them. Historically it was Rani Lakshmi Bai of Jhansi who sowed the first seeds of rebellion towards almost a century long battle for the freedom of India. Our history is replete with stories of many such brave women who have walked the untrodden path. Lives of Nagamma the leader of Palanati Seema, Brahma Naidu’s mother Seelamma, Tribal king Yerukalaraju’s mother vii Rani Rudrama Devi Women Pioneers Kuntala Devi, Erramma Kanasanamma, the lady who contributed to resurrection of the Kakatiya clan, the valorous Gond Queen Rani Durgavati 1524-1564 AD, the Muslim woman warrior Chand Bibi 1550-1599 AD, great ruler and Queen of the Malwa kingdom Devi Ahilyabai Holker 1725-1795 AD and the first and only woman ruler to sit on the throne of Delhi Sultanate Razia Sultan 1236 AD, are women who have excelled in every aspect of their lives and inspired us. -

LIST of FARMS REGISTERED in EAST GODAVARI DISTRICT * Valid for 5 Years from the Date of Issue
LIST OF FARMS REGISTERED IN EAST GODAVARI DISTRICT * Valid for 5 Years from the Date of Issue. Address Farm Address S.No. Registration No. Name Father's / Husband's name Survey Number Issue date * Village / P.O. Mandal District Mandal Revenue Village Karri Venkat C/o D Divakara Reddy, 116/3; 114/5,7,11; 1 AP-II-2007(01621) Krishna Reddy Shri Venkat Reddy Gollala Mamidada Pedapudi Mandal East Godavari District Pedapudi Pedapudi 110/1 26.11.2007 Medapati Sura C/o D Divakara Reddy, 120/1A, 122/3 to 5; 2 AP-II-2007(01622) Reddy Shri Rama Reddy Gollala Mamidada Pedapudi Mandal East Godavari District Pedapudi Pedapudi 111/1 26.11.2007 Katta Veera C/o D Divakara Reddy, 3 AP-II-2007(01623) Raghavalu Shri Venkanna Gollala Mamidada Pedapudi Mandal East Godavari District Pedapudi Pedapudi 122/2 26.11.2007 Boddupalli Appa C/o D Divakara Reddy, 4 AP-II-2007(01624) Rao Shri Appala Swamy Gollala Mamidada Pedapudi Mandal East Godavari District Pedapudi Pedapudi 112/1,4,5,6,2,8,10 26.11.2007 112/9, 111/4, 112/3, C/o D Divakara Reddy, 112/7, 111/2, 112/1, 5 AP-II-2007(01625) Bera Setha Ramulu Shri Suryanarayana Gollala Mamidada Pedapudi Mandal East Godavari District Pedapudi Pedapudi 120/B; 122/1 26.11.2007 Palepu Venkat C/o D Divakara Reddy, 111/3, 111/5, 111/6, 6 AP-II-2007(01626) Ramana Shri Chinna Appala Swamy Gollala Mamidada Pedapudi Mandal East Godavari District Pedapudi Pedapudi 111/7 26.11.2007 Dwarampudi C/o D Divakara Reddy, 7 AP-II-2007(01627) Divakara Reddy Shri Appa Reddy Gollala Mamidada Pedapudi Mandal East Godavari District Pedapudi Pedapudi