The Vegetation of the Western Australian Deserts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Additional Information
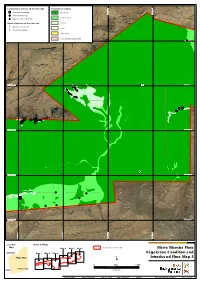
Current Survey Introduced Flora Records Vegetation Condition *Acetosa vesicaria Excellent 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 -

A Phylogeny of the Hubbardochloinae Including Tetrachaete (Poaceae: Chloridoideae: Cynodonteae)
Peterson, P.M., K. Romaschenko, and Y. Herrera Arrieta. 2020. A phylogeny of the Hubbardochloinae including Tetrachaete (Poaceae: Chloridoideae: Cynodonteae). Phytoneuron 2020-81: 1–13. Published 18 November 2020. ISSN 2153 733 A PHYLOGENY OF THE HUBBARDOCHLOINAE INCLUDING TETRACHAETE (CYNODONTEAE: CHLORIDOIDEAE: POACEAE) PAUL M. PETERSON AND KONSTANTIN ROMASCHENKO Department of Botany National Museum of Natural History Smithsonian Institution Washington, D.C. 20013-7012 [email protected]; [email protected] YOLANDA HERRERA ARRIETA Instituto Politécnico Nacional CIIDIR Unidad Durango-COFAA Durango, C.P. 34220, México [email protected] ABSTRACT The phylogeny of subtribe Hubbardochloinae is revisited, here with the inclusion of the monotypic genus Tetrachaete, based on a molecular DNA analysis using ndhA intron, rpl32-trnL, rps16 intron, rps16- trnK, and ITS markers. Tetrachaete elionuroides is aligned within the Hubbardochloinae and is sister to Dignathia. The biogeography of the Hubbardochloinae is discussed, its origin likely in Africa or temperate Asia. In a previous molecular DNA phylogeny (Peterson et al. 2016), the subtribe Hubbardochloinae Auquier [Bewsia Gooss., Dignathia Stapf, Gymnopogon P. Beauv., Hubbardochloa Auquier, Leptocarydion Hochst. ex Stapf, Leptothrium Kunth, and Lophacme Stapf] was found in a clade with moderate support (BS = 75, PP = 1.00) sister to the Farragininae P.M. Peterson et al. In the present study, Tetrachaete elionuroides Chiov. is included in a phylogenetic analysis (using ndhA intron, rpl32- trnL, rps16 intron, rps16-trnK, and ITS DNA markers) in order to test its relationships within the Cynodonteae with heavy sampling of species in the supersubtribe Gouiniodinae P.M. Peterson & Romasch. Chiovenda (1903) described Tetrachaete Chiov. with a with single species, T. -

Supplementary Materialsupplementary Material
10.1071/BT13149_AC © CSIRO 2013 Australian Journal of Botany 2013, 61(6), 436–445 SUPPLEMENTARY MATERIAL Comparative dating of Acacia: combining fossils and multiple phylogenies to infer ages of clades with poor fossil records Joseph T. MillerA,E, Daniel J. MurphyB, Simon Y. W. HoC, David J. CantrillB and David SeiglerD ACentre for Australian National Biodiversity Research, CSIRO Plant Industry, GPO Box 1600 Canberra, ACT 2601, Australia. BRoyal Botanic Gardens Melbourne, Birdwood Avenue, South Yarra, Vic. 3141, Australia. CSchool of Biological Sciences, Edgeworth David Building, University of Sydney, Sydney, NSW 2006, Australia. DDepartment of Plant Biology, University of Illinois, Urbana, IL 61801, USA. ECorresponding author. Email: [email protected] Table S1 Materials used in the study Taxon Dataset Genbank Acacia abbreviata Maslin 2 3 JF420287 JF420065 JF420395 KC421289 KC796176 JF420499 Acacia adoxa Pedley 2 3 JF420044 AF523076 AF195716 AF195684; AF195703 Acacia ampliceps Maslin 1 KC421930 EU439994 EU811845 Acacia anceps DC. 2 3 JF420244 JF420350 JF419919 JF420130 JF420456 Acacia aneura F.Muell. ex Benth 2 3 JF420259 JF420036 JF420366 JF419935 JF420146 KF048140 Acacia aneura F.Muell. ex Benth. 1 2 3 JF420293 JF420402 KC421323 JQ248740 JF420505 Acacia baeuerlenii Maiden & R.T.Baker 2 3 JF420229 JQ248866 JF420336 JF419909 JF420115 JF420448 Acacia beckleri Tindale 2 3 JF420260 JF420037 JF420367 JF419936 JF420147 JF420473 Acacia cochlearis (Labill.) H.L.Wendl. 2 3 KC283897 KC200719 JQ943314 AF523156 KC284140 KC957934 Acacia cognata Domin 2 3 JF420246 JF420022 JF420352 JF419921 JF420132 JF420458 Acacia cultriformis A.Cunn. ex G.Don 2 3 JF420278 JF420056 JF420387 KC421263 KC796172 JF420494 Acacia cupularis Domin 2 3 JF420247 JF420023 JF420353 JF419922 JF420133 JF420459 Acacia dealbata Link 2 3 JF420269 JF420378 KC421251 KC955787 JF420485 Acacia dealbata Link 2 3 KC283375 KC200761 JQ942686 KC421315 KC284195 Acacia deanei (R.T.Baker) M.B.Welch, Coombs 2 3 JF420294 JF420403 KC421329 KC955795 & McGlynn JF420506 Acacia dempsteri F.Muell. -

Ngaanyatjarra Central Ranges Indigenous Protected Area
PLAN OF MANAGEMENT for the NGAANYATJARRA LANDS INDIGENOUS PROTECTED AREA Ngaanyatjarra Council Land Management Unit August 2002 PLAN OF MANAGEMENT for the Ngaanyatjarra Lands Indigenous Protected Area Prepared by: Keith Noble People & Ecology on behalf of the: Ngaanyatjarra Land Management Unit August 2002 i Table of Contents Notes on Yarnangu Orthography .................................................................................................................................. iv Acknowledgements........................................................................................................................................................ v Cover photos .................................................................................................................................................................. v Abbreviations ................................................................................................................................................................. v Summary.................................................................................................................................................................................... 1 1 Introduction ....................................................................................................................................................................... 2 1.1 Background ............................................................................................................................................................... -

Willi Orchids
growers of distinctively better plants. Nunured and cared for by hand, each plant is well bred and well fed in our nutrient rich soil- a special blend that makes your garden a healthier, happier, more beautiful place. Look for the Monrovia label at your favorite garden center. For the location nearest you, call toll free l-888-Plant It! From our growing fields to your garden, We care for your plants. ~ MONROVIA~ HORTICULTURAL CRAFTSMEN SINCE 1926 Look for the Monrovia label, call toll free 1-888-Plant It! co n t e n t s Volume 77, Number 3 May/June 1998 DEPARTMENTS Commentary 4 Wild Orchids 28 by Paul Martin Brown Members' Forum 5 A penonal tour ofplaces in N01,th America where Gaura lindheimeri, Victorian illustrators. these native beauties can be seen in the wild. News from AHS 7 Washington, D . C. flower show, book awards. From Boon to Bane 37 by Charles E. Williams Focus 10 Brought over f01' their beautiful flowers and colorful America)s roadside plantings. berries, Eurasian bush honeysuckles have adapted all Offshoots 16 too well to their adopted American homeland. Memories ofgardens past. Mock Oranges 41 Gardeners Information Service 17 by Terry Schwartz Magnolias from seeds, woodies that like wet feet. Classic fragrance and the ongoing development of nell? Mail-Order Explorer 18 cultivars make these old favorites worthy of considera Roslyn)s rhodies and more. tion in today)s gardens. Urban Gardener 20 The Melting Plot: Part II 44 Trial and error in that Toddlin) Town. by Susan Davis Price The influences of African, Asian, and Italian immi Plants and Your Health 24 grants a1'e reflected in the plants and designs found in H eading off headaches with herbs. -

Grass Genera in Townsville
Grass Genera in Townsville Nanette B. Hooker Photographs by Chris Gardiner SCHOOL OF MARINE and TROPICAL BIOLOGY JAMES COOK UNIVERSITY TOWNSVILLE QUEENSLAND James Cook University 2012 GRASSES OF THE TOWNSVILLE AREA Welcome to the grasses of the Townsville area. The genera covered in this treatment are those found in the lowland areas around Townsville as far north as Bluewater, south to Alligator Creek and west to the base of Hervey’s Range. Most of these genera will also be found in neighbouring areas although some genera not included may occur in specific habitats. The aim of this book is to provide a description of the grass genera as well as a list of species. The grasses belong to a very widespread and large family called the Poaceae. The original family name Gramineae is used in some publications, in Australia the preferred family name is Poaceae. It is one of the largest flowering plant families of the world, comprising more than 700 genera, and more than 10,000 species. In Australia there are over 1300 species including non-native grasses. In the Townsville area there are more than 220 grass species. The grasses have highly modified flowers arranged in a variety of ways. Because they are highly modified and specialized, there are also many new terms used to describe the various features. Hence there is a lot of terminology that chiefly applies to grasses, but some terms are used also in the sedge family. The basic unit of the grass inflorescence (The flowering part) is the spikelet. The spikelet consists of 1-2 basal glumes (bracts at the base) that subtend 1-many florets or flowers. -
Acacia Synchronicia Maslin
WATTLE Acacias of Australia Acacia synchronicia Maslin Source: W orldW ideW attle ver. 2. Published at: w w w .w orldw idew attle.com B.R. Maslin Source: W orldW ideW attle ver. 2. Source: Australian Plant Image Index Image courtesy of Northern Territory Herbarium Published at: w w w .w orldw idew attle.com (dig.15818). B.R. Maslin ANBG © M. Fagg, 2009 Source: W orldW ideW attle ver. 2. Source: W orldW ideW attle ver. 2. Source: W orldW ideW attle ver. 2. Published at: w w w .w orldw idew attle.com Published at: w w w .w orldw idew attle.com Published at: w w w .w orldw idew attle.com B.R. Maslin B.R. Maslin B.R. Maslin Source: W orldW ideW attle ver. 2. Published at: w w w .w orldw idew attle.com B.R. Maslin Source: W orldW ideW attle ver. 2. Published at: w w w .w orldw idew attle.com Kym Brennan Source: W orldW ideW attle ver. 2. Source: W orldW ideW attle ver. 2. Source: W orldW ideW attle ver. 2. Published at: w w w .w orldw idew attle.com Published at: w w w .w orldw idew attle.com Published at: w w w .w orldw idew attle.com B.R. Maslin B.R. Maslin B.R. Maslin Source: Australian Plant Image Index (dig.15819). ANBG © M. Fagg, 2009 Source: W orldW ideW attle ver. 2. Source: W orldW ideW attle ver. 2. Source: W orldW ideW attle ver. 2. Published at: w w w .w orldw idew attle.com Published at: w w w .w orldw idew attle.com Published at: w w w .w orldw idew attle.com J. -

Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(S): Grass Phylogeny Working Group, Nigel P
Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(s): Grass Phylogeny Working Group, Nigel P. Barker, Lynn G. Clark, Jerrold I. Davis, Melvin R. Duvall, Gerald F. Guala, Catherine Hsiao, Elizabeth A. Kellogg, H. Peter Linder Source: Annals of the Missouri Botanical Garden, Vol. 88, No. 3 (Summer, 2001), pp. 373-457 Published by: Missouri Botanical Garden Press Stable URL: http://www.jstor.org/stable/3298585 Accessed: 06/10/2008 11:05 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=mobot. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit organization founded in 1995 to build trusted digital archives for scholarship. We work with the scholarly community to preserve their work and the materials they rely upon, and to build a common research platform that promotes the discovery and use of these resources. For more information about JSTOR, please contact [email protected]. -

Onslow Metals Turtle and Range Deposits Level 1 Flora Survey June
Onslow Metals Turtle and Range Deposits Level 1 Flora Survey June 2011 Dr. Belinda Newman (Author) Newman Environmental 130 Riverview Ave South Guildford WA, 6055 Ph: 0433768578 ABN: 85 437 176 819 Email: [email protected] This page has been left intentionally blank Executive Summary Onslow Metals propose to conduct drilling and exploration activities and activate a quarry over the Turtle and Range tenements, approximately 80km south of Onslow. A reconnaissance survey and level one flora report were prepared for the site. The site has had some historic drilling and exploration activities, resulting in localised vegetation disturbance. Turtle Deposit tenement is 50ha in size and Range tenement is 120ha in size. The area that Onslow Metals will potentially disturb is approximately 15ha. Searches of the DEC databases revealed that two Priority flora were known to occur within the vicinity of the site. Neither of these species was observed during the reconnaissance survey. Following a review of other surveys within the area, and the habitat preferences of these two species, it was deemed that these species do not occur on the Onslow Metals tenements. The vegetation at the site ranges in condition from Good to Poor condition. Vegetation which is in Good condition has the potential to deteriorate over time with two introduced species recorded for the site having a „high‟ rating as an environmental weed. The site does not contain any Threatened or Priority Ecological Communities and the vegetation units are well represented in a regional context. As there are no Threatened or Priority flora, the vegetation and flora of the site has no conservation significance. -

Kingdom Class Family Scientific Name Common Name I Q a Records
Kingdom Class Family Scientific Name Common Name I Q A Records plants monocots Poaceae Paspalidium rarum C 2/2 plants monocots Poaceae Aristida latifolia feathertop wiregrass C 3/3 plants monocots Poaceae Aristida lazaridis C 1/1 plants monocots Poaceae Astrebla pectinata barley mitchell grass C 1/1 plants monocots Poaceae Cenchrus setigerus Y 1/1 plants monocots Poaceae Echinochloa colona awnless barnyard grass Y 2/2 plants monocots Poaceae Aristida polyclados C 1/1 plants monocots Poaceae Cymbopogon ambiguus lemon grass C 1/1 plants monocots Poaceae Digitaria ctenantha C 1/1 plants monocots Poaceae Enteropogon ramosus C 1/1 plants monocots Poaceae Enneapogon avenaceus C 1/1 plants monocots Poaceae Eragrostis tenellula delicate lovegrass C 2/2 plants monocots Poaceae Urochloa praetervisa C 1/1 plants monocots Poaceae Heteropogon contortus black speargrass C 1/1 plants monocots Poaceae Iseilema membranaceum small flinders grass C 1/1 plants monocots Poaceae Bothriochloa ewartiana desert bluegrass C 2/2 plants monocots Poaceae Brachyachne convergens common native couch C 2/2 plants monocots Poaceae Enneapogon lindleyanus C 3/3 plants monocots Poaceae Enneapogon polyphyllus leafy nineawn C 1/1 plants monocots Poaceae Sporobolus actinocladus katoora grass C 1/1 plants monocots Poaceae Cenchrus pennisetiformis Y 1/1 plants monocots Poaceae Sporobolus australasicus C 1/1 plants monocots Poaceae Eriachne pulchella subsp. dominii C 1/1 plants monocots Poaceae Dichanthium sericeum subsp. humilius C 1/1 plants monocots Poaceae Digitaria divaricatissima var. divaricatissima C 1/1 plants monocots Poaceae Eriachne mucronata forma (Alpha C.E.Hubbard 7882) C 1/1 plants monocots Poaceae Sehima nervosum C 1/1 plants monocots Poaceae Eulalia aurea silky browntop C 2/2 plants monocots Poaceae Chloris virgata feathertop rhodes grass Y 1/1 CODES I - Y indicates that the taxon is introduced to Queensland and has naturalised. -

DRAFT 25/10/90; Plant List Updated Oct. 1992; Notes Added June 2021
DRAFT 25/10/90; plant list updated Oct. 1992; notes added June 2021. PRELIMINARY REPORT ON THE CONSERVATION VALUES OF OPEN COUNTRY PADDOCK, BOOLARDY STATION Allan H. Burbidge and J.K. Rolfe INTRODUCTION Boolardy Station is situated about 150 km north of Yalgoo and 140 km west-north-west of Cue, in the Shire of Murchison, Western Australia. Open Country Paddock (about 16 000 ha) is in the south-east corner of the station, at 27o05'S, 116o50'E. The most prominent named feature is Coolamooka Hill, near the eastern boundary of the paddock. There are no conservation reserves in this region, although there are some small reserves set aside for various other purposes. Previous biological data for the station consist of broad scale vegetation mapping and land system mapping. Beard (1976) mapped the entire Murchison region at 1: 1 000 000. The Open Country Paddock area was mapped as supporting mulga woodlands and shrublands. More detailed mapping of land system units for rangeland assessment purposes has been carried out more recently at a scale of 1: 40 000 (Payne and Curry in prep.). Seven land systems were identified in open Country Paddock (Fig. 1). Apart from these studies, no detailed biological survey work appears to have been done in the area. Open Country Paddock has been only lightly grazed by domestic stock because of the presence of Kite-leaf Poison (Gastrolobium laytonii) and a lack of fresh water. Because of this and the generally good condition of the paddock and presence of a wide range of plant species, P.J. -

Volume 5 Pt 3
Conservation Science W. Aust. 7 (1) : 153–178 (2008) Flora and Vegetation of the banded iron formations of the Yilgarn Craton: the Weld Range ADRIENNE S MARKEY AND STEVEN J DILLON Science Division, Department of Environment and Conservation, Wildlife Research Centre, PO Box 51, Wanneroo WA 6946. Email: [email protected] ABSTRACT A survey of the flora and floristic communities of the Weld Range, in the Murchison region of Western Australia, was undertaken using classification and ordination analysis of quadrat data. A total of 239 taxa (species, subspecies and varieties) and five hybrids of vascular plants were collected and identified from within the survey area. Of these, 229 taxa were native and 10 species were introduced. Eight priority species were located in this survey, six of these being new records for the Weld Range. Although no species endemic to the Weld Range were located in this survey, new populations of three priority listed taxa were identified which represent significant range extensions for these taxa of conservation significance. Eight floristic community types (six types, two of these subdivided into two subtypes each) were identified and described for the Weld Range, with the primary division in the classification separating a dolerite-associated floristic community from those on banded iron formation. Floristic communities occurring on BIF were found to be associated with topographic relief, underlying geology and soil chemistry. There did not appear to be any restricted communities within the landform, but some communities may be geographically restricted to the Weld Range. Because these communities on the Weld Range are so closely associated with topography and substrate, they are vulnerable to impact from mineral exploration and open cast mining.