Modelling and Optimization of Ethanol Production from Cassava (Manihot Esculenta) Otaraku I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
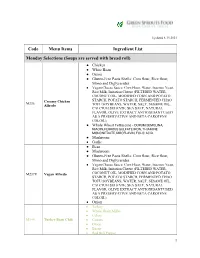
Code Menu Items Ingredient List Monday Selections (Soups Are
Updated 6.15.2021 Code Menu Items Ingredient List Monday Selections (Soups are served with bread roll) ● Chicken ● White Bean ● Onion ● Gluten-Free Pasta Shells: Corn flour, Rice flour, Mono and Diglycerides ● Vegan Cheese Sauce: Corn Flour, Water, Inactive Yeast, Rice Milk, Imitation Cheese (FILTERED WATER, COCONUT OIL, MODIFIED CORN AND POTATO Creamy Chicken STARCH, POTATO STARCH, FERMENTED CHAO M236 Alfredo TOFU SOYBEANS, WATER, SALT, SESAME OIL, CALCIUM SULFATE, SEA SALT, NATURAL FLAVOR, OLIVE EXTRACT ANTIOXIDANT USED AS A PRESERVATIVE AND BETA CAROTENE COLOR.) ● Whole Wheat Fettuccine - DURUM SEMOLINA, NIACIN, FERROUS SULFATE IRON, THIAMINE MONONITRATE, RIBOFLAVIN, FOLIC ACID. ● Mushroom ● Garlic ● Bean ● Mushroom ● Gluten-Free Pasta Shells: Corn flour, Rice flour, Mono and Diglycerides ● Vegan Cheese Sauce: Corn Flour, Water, Inactive Yeast, Rice Milk, Imitation Cheese (FILTERED WATER, M237V Vegan Alfredo COCONUT OIL, MODIFIED CORN AND POTATO STARCH, POTATO STARCH, FERMENTED CHAO TOFU SOYBEANS, WATER, SALT, SESAME OIL, CALCIUM SULFATE, SEA SALT, NATURAL FLAVOR, OLIVE EXTRACT ANTIOXIDANT USED AS A PRESERVATIVE AND BETA CAROTENE COLOR.) ● Onion • Turkey • Whole Grain Millet • Celery M144 Turkey Bean Chili • Carrots • Onion • Beans • Red Bell Pepper 1 • Corn • Tomato Sauce • Cumin • Bay Leaves • Chili Powder • Paprika • Chicken Broth ● Cheddar Cheese • Tofu • Whole Grain Millet • Celery • Carrots • Onion • Beans • Red Bell Pepper M145V Tofu Bean Chili • Corn • Tomato Sauce • Cumin • Bay Leaves • Chili Powder • Paprika • Chicken Broth -

What Makes Premium Cassava Flour Special?
2018 E-zine Coverage What Makes Premium Cassava Flour Special? Manufacturers of baked goods with the development of adopted starch manufacturing and snack foods differentiate delicious, high-quality baked technology and since produced in the crowded marketplace goods and snack foods. and sold only tapioca starch by offering specialty products and a number of them that speak to today’s FBN: What is cassava? continued to refer to this consumers’ wants and needs. new ingredient as tapioca Some of the most frequently Mr. Festejo: Cassava, also flour. Tapioca flour, or more requested products are those commonly known as yucca or appropriately, cassava flour, is with gluten-free, grain-free and manioc, is a perennial woody still produced and consumed non-GMO claims. shrub with an edible root that in tropical countries where the grows in tropical and subtropical cassava plant is indigenously Food Business News spoke with areas of the world. While many grown. However, the quality and Mel Festejo, COO of AKFP to food professionals are familiar characteristics of the flour are learn how Premium Cassava with and have used tapioca adapted only to native dishes Flour, alone or in a mix, assists starches and like derivatives, few in these countries. These local know that tapioca’s raw material cassava flours are not well is the root of the cassava plant. suited for use in gluten-free Tapioca or cassava starch is baked goods and snack foods. produced by the extraction of Our proprietary technology is only the starch component of making cassava flour a highly the root. Tapioca or cassava suitable functional, nutritional, flour, on the other hand, requires clean-label ingredient for gluten- the processing of the whole free and even grain-free baking peeled root. -

Around the World in 80 Food Trucks 1 Preview
Lobster mac ‘n’ cheese, BOB’s Lobster 26 Buttermilk-fried-chicken biscuit OCEANIA SOUTH AMERICA Newport bowl with tofu, Califarmication 144 Great balls of fire pork & beef meatballs, sandwiches, Humble Pie 56 Detox açai bowl, Açai Corner 82 Peruvian sacha taco, Hit ‘n Run 110 BEC sandwich, Paperboy 146 contents The Bowler 28 Super cheddar burger, Paneer 58 Carolina smoked pulled pork sandwich, The Cajacha burger, Lima Sabrosa 112 The Viet banh mi, Chicago Lunchbox 148 Goat’s cheese, honey & walnut Turkey burger, Street Chefs 60 Sneaky Pickle 84 Yakisoba bowl, Luca China 114 Blue cheese slaw, Banjo's 150 Introduction 5 toasted sandwich, The Cheese Truck 30 Forest pancake, Belki&Uglevody 62 The Bahh lamb souvlaki, Argentine sandwich, El Vagabundo 116 Old-fashioned peach cake, The Peach Truck 152 Jerusalem spiced chicken, Laffa 32 Greek Street Food 86 Uruguayan flan, Merce Daglio Sweet Truck 118 Alabama tailgaters, Fidel Gastro’s 154 EUROPE Fried chicken, Mother Clucker 34 AFRICA & MIDDLE EAST Mr Chicken burger, Mr Burger 88 Tapioca pancake, Tapí Tapioca 120 Nanner s’more, Meggrolls 156 Spicy Killary lamb samosas, Chicken, chilli & miso gyoza, Rainbo 36 South African mqa, 4Roomed eKasi Culture 64 Tuscan beef ragu, Pasta Face 90 Red velvet cookies, Misunderstood Heron 6 Asparagus, ewe’s cheese, mozzarella & Asian bacon burger, Die Wors Rol 66 The Fonz toastie, Toasta 92 NORTH AMERICA Captain Cookie and the Milk Man 158 San Francisco langoustine roll, hazelnut pizza, Well Kneaded 38 Chicken tikka, Hangry Chef 68 Butter paneer masala, Yo India -

5 Minced and Moist Food Level 5
Name: ………………………………………………………… Tel: 01225 824333 Date: ……………………………………………..…………… Speech & Language Therapist: …………………………. Minced and Moist Food 5 Level 5 5 General description: Food is soft, tender and moist. Needs very little chewing and no biting. Food has been mashed up before serving. Small lumps visible within the food (no greater than 4mm in size). Lumps are easy to squash with the tongue. It usually requires a smooth sauce, gravy or custard, which should be very thick. No mixed (thick - thin) textures e.g. cereal in milk, mince in gravy, dried fruit in sponge. No thin loose fluid. No hard, tough, chewy (toffee), fibrous (dry roasted meat), stringy (pineapple, celery), dry, crispy, crunchy (toast, flaky pastry) or crumbly bits (bread crusts, dry biscuits). No pips, seeds, pith, membrane, e.g. citrus fruit, tomatoes. No skins or outer shells e.g. on peas, grapes. No husks. No skin e.g. on sausages (use skinless ones), bone or gristle. No round or long-shaped foods e.g. grapes, sweets. No hard chunks e.g. pieces of apple. No sticky foods e.g. cheese chunks, marshmallows. Check before serving/eating: • No hard pieces, crusts or skins have formed during cooking/heating/standing. • Fluid/gravy/sauce/custard in or on the food has not thinned out or separated off. Please turn over for more information Based on Dysphagia Diet Food Texture Descriptors March 2012 and IDDSI (International Dysphagia Diet Standardisation Initiative) Framework – April 2018 Minced and Moist Food 5 Level 5 5 Meat • Must be finely minced – pieces approximately 4mm. No hard bits of mince. • Serve in a very thick, smooth sauce or gravy. -

Growth of Raphia Farinifera and Elaeis Guineensis Wine Yeast on Cassava (Manihot Esculenta ) and Poultry Manure Media
Nyerhovwo J. Tonukari et al. NISEB Journal Vol. 14, No. 3, September, 2014 1595-6938/2014 Printed in Nigeria (2014) Nigerian Society for Experimental Biology http://www.nisebjournal.org Growth of Raphia farinifera and Elaeis guineensis Wine Yeast on Cassava (Manihot esculenta ) and Poultry Manure Media 1,2 1,2 1,2 2 2 Nyerhovwo J. Tonukari *, Oghenetega J. Avwioroko , Theresa Ezedom , Ufuoma Edema , Linda Eraga and Akpovwehwee A. Anigboro 1 1 Department of Biochemistry, Faculty of Science, Delta State University, Abraka, Nigeria. 2African Research Laboratories, Otorho-Agbon (Isiokolo), Delta State, Nigeria. Abstract The aim of this research was to determine the suitability of cassava (Manihot esculenta) and poultry manure (locally available resources) as substrates for the production of raffia and palm wine yeasts. Yeast growth was estimated as a measure of their medium’s optical density at 600 nm (OD 600 nm ) after 36 h of growth. The various media used were formed from different combinations of cassava flour and poultry manure. The results obtained showed that the growth of the palm wine (Elaeis guineensis Jacq.) yeast in the locally formulated media 3:2, 4:1, 5:0 [poultry manure (PM)(g)/ cassava flour (CS)(g)], and yeast extract-peptone dextrose medium (YPD) was 2.101, 2.830, 3.131 and 2.057 respectively. The OD 600 nm measurement of the growth of the raffia wine (Raphia farinifera) yeast was 2.912, 2.859, 4.000, and 2.110 respectively in the same media above. This shows that formulations 3:2, 4:1, and 5:0 [PM (g)/CS (g)] are highly suitable for the growth of palm wine and raffia wine yeasts when compared with their growth in the standard medium (YPD). -

Flottweg Centrifuge Technology for Processing Tapioca / Cassava / Manioc Starch
FLOTTWEG CENTRIFUGE TECHNOLOGY FOR PROCESSING TAPIOCA / CASSAVA / MANIOC STARCH High Quality Separation Technology For Your Profit FLOTTWEG TECHNOLOGY FOR THE PRODUCTION OF TAPIOCA STARCH Efficient Centrifuge Technology Made by Flottweg Tapioca starch, also known as manioc or cas- Flottweg Separation Technology, however, satisfies varying customer sava starch is extracted from the roots of the cas- and consumer needs by using high quality separation technology sava plant. For example, to obtain one kilogram regardless of prevailing conditions, industrial sectors, or scale of fac- of tapioca starch, approximately 4.4 kilograms of tories. cassava roots are required (25 percent of starch content). The cassava plant has spread through- out Africa and South America; however its use Flottweg Separation Technology – for culinary purposes has become important and Your Benefits has spread all over the world. Additionally, be- sides corn starch, tapioca starch has evolved as • The Flottweg Decanter convinces customers all over the one of the most important sources of starch world with efficiency, durability and high quality, especially across the globe. when it comes to the processing of starch. • Flottweg provides almost 60 years of outstanding Often used as a thickener, tapioca possesses a experience in the field of mechanical separation. neutral flavor and consists of almost 100 percent • Plant and system construction for satisfying individual of carbohydrates. Moreover, tapioca is protein customer requirements as well as perfectly adjusted and fat free and does not contain gluten which processes symbolizes its importance for today's industry • The Flottweg Simp Drive® enables high throughput sectors. As a result, the demand for tapioca performance due to high bowl speed and precisely starch has been increasing, generating applica- adjusted differential speed. -

Heavy Metals in Tapioca Locally Processed by Sun-Drying Method Along Enugu-Abakaliki Highway in Ezilo, Ebonyi State, Nigeria
IOSR Journal of Environmental Science, Toxicology and Food Technology (IOSR-JESTFT) e-ISSN: 2319-2402,p- ISSN: 2319-2399.Volume 10, Issue 7 Ver. II (July 2016), PP 85-87 www.iosrjournals.org Heavy Metals In Tapioca Locally Processed By Sun-Drying Method Along Enugu-Abakaliki Highway In Ezilo, Ebonyi State, Nigeria Oti, Wilberforce J. O Department of Industrial Chemistry, Ebonyi State University, Private Mail Bag 053, Abakaliki, Ebonyi State, Nigeria. Abstract: Samples of flakes of cassava (Manihot esculenta) popularly known as tapioca from the same source were divided into two and one group was processed by sun-drying along Ezillo Road side while the other by in- door spreading served as control. Atomic Absorption Spectrophotometer model AGP 210 Bulk scientific was used to determine the levels of heavy metals in the tapioca samples. The result (in ppm) revealed the following concentration: Zn (11.13 ± 0.08); Cd (2.50 ± 0.03); As (0.15 ± 0.01); Pb (0.53 ± 0.02) and Cr (3.00 ± 0.04) for sundried tapioca while that of the results (in ppm) of the control (indoor tapioca) were Zn (13.38 ± 0.11); Cd (0.06 ± 0.01); As (0.13 ± 0.01) ; Pb (0.35 ± 0.01) and Cr (1.75 ± 0.03). The levels of heavy metals in samples of sundried tapioca were generally higher than those processed in-doors. Statistical analysis revealed there was no significant difference (p > 0.05) in the mean concentration of Zn, As and Cr in sundried samples when compared with the control, while the levels of Pb and Cd that were significantly (p<0.05). -

6 Quick and Simple Tofu Recipes Well-Balanced, Whole-Food, Plant Based
6 quick and simple tofu recipes Well-balanced, whole-food, plant based Follow me for more family recipes: IG: @at.my.table Blog: www.at-my-table.ca -1 block of extra firm tofu, cubed. -1 block medium firm tofu, cubed -2 clove garlic, minced -2 blocks of sunrise soy firm tofu -2 cups of cooked chickpeas -1 tbs toasted sesame oil -1/4 cup soy sauce or aminos -1 onion, chopped -1/2 cup veggie broth -Juice from 1/2 lime -3 garlic cloves, minced -1 tsp ground ginger -1 cup nutritional yeast -4 roma tomatoes -2 tbs maple syrup ● -1 tbs each of curry powder, -1 tbs rice vinegar cumin, turmeric, and coriander. -1 tbs lime juice -¼ tsp each salt and pepper -1 tbs tapioca or arrowroot flour -2 cups of veggie broth and -2 tbs water -2 limes, juiced -2 tbs toasted sesame seeds -1 can of coconut milk 1.Press your tofu before you start. Simple remove from -1 whole cauliflower, cut small packaging, place on a large plate. Then place a smaller 1. Over a medium hot pan add the sesame oil to the pan plate on top and add 2 or 3 cookbooks. Leave for about 1.In a large pot over medium high heat saute the onions and lightly brown your cubed tofu pieces. Add a little 1 hour. in some olive oil. Once soft add in the garlic, tomatoes, water and turn heat down if it starts to burn. 2.Then cut into cubes and place in a large bowl. Add in and all the spices and cook for another 2 minutes, until 2. -

A Cinnamon and Benzoate Free Diet for Orofacial Granulomatosis
May 2015 A cinnamon and benzoate free diet for orofacial granulomatosis: Orofacial granulomatosis (OFG) is a condition which affects mainly the mouth and lips. Swelling and redness are the most common symptoms but other symptoms such as mouth ulcers and cracked lips can occur too. The cause is not known but a cinnamon and benzoate free diet helps 70% of people with OFG. Avoiding foods which contain cinnamon and benzoates may help your oral symptoms. You should try and follow this diet for 12 weeks and monitor any improvements in your symptoms diary. Keep to fresh or home cooked food where possible. If you are unsure whether a food or drink may contain cinnamon or benzoate, it is best to avoid it. It is important that you read the labels of any manufactured or prepared foods you consume. 1 Page 2 of 13 Cinnamon Cinnamon is a natural substance, which because it is used in very small quantities does not always have to be stated on food labels. Look for the word spices, spice extracts, ground cinnamon, mixed spice, cinnamon oil, cinnamal or cinnamic aldehyde on food labels. Benzoates Most benzoates are added to food and drinks as a preservative. They are commonly added to fizzy drinks and processed foods. High levels of benzoates may also occur naturally in certain foods. Benzoates includes any of these preservatives: E210 or Benzoic acid E211 or Sodium benzoate E212 or Potassium benzoate E213 or Calcium benzoate E214 or Ethyl 4-hydroxybenzoate or Ethyl para-hydroxybenzoate E215 or Ethyl 4-hydroxybenzoate, sodium salt or sodium ethyl para-hydroxybenzoate *E216 or Propyl 4-hydroxybenzoate or Propyl para-hydroxybenzoate *E217 or Propyl 4-hydroxybenzoate, sodium salt or sodium para-hydroxybenzoate E218 or Methyl 4-hydroxybenzoate or Methyl para-hydroxybenzoate E219 or Methyl 4-hydroxybenzoate, sodium salt or sodium methyl-hydroxybenzoate *banned in foods produced within the European Union but may be found in imported products. -

Indonesian Food Culture Mapping: a Starter Contribution to Promote Indonesian Culinary Tourism Serli Wijaya
Wijaya Journal of Ethnic Foods (2019) 6:9 Journal of Ethnic Foods https://doi.org/10.1186/s42779-019-0009-3 REVIEW ARTICLE Open Access Indonesian food culture mapping: a starter contribution to promote Indonesian culinary tourism Serli Wijaya Abstract The food culture of Indonesia is shaped by several factors such as nature, history, and culture. With its enormous geographic and cultural diversity across the archipelagos, it is evident that Indonesian cuisine is rich in variety and taste. As such, food can be utilised as a strategic means to boost the tourism industry of the country. In the past 5 years, the Indonesian government has given a great support for the development of culinary tourism as one special interest tourism sector that is promoted extensively to the international market. Promoting Indonesian culinary tourism should not be merely exposing the ample varieties of the traditional food that Indonesia has, but more importantly, telling the market about the socio-cultural values behind the food itself. This study aimed to portray how Indonesian food culture has been shaped, developed, and held as the value embedded in the society and has been passed from one generation to the next. For the purpose of the study, a range of literature from journal articles, books, archives, magazines, and articles to Internet sources that are relevant to Indonesian culinary discussions was reviewed. Keywords: Food culture, Indonesian cuisine, Culinary tourism Introduction international visitors [5]. There are thousands of local Food plays a significant part in all aspects of human life, foods which potentially offer a strong focal point to por- including from fulfilling basic physiological needs to tray Indonesia as a tourism destination. -

The Purpose of a Low Fiber, Low Residue Diet Is to Minimize Stool Output
Digestive Health Center Nutrition Services Low Fiber Diet Fiber is a component of complex carbohydrates that the body cannot digest, is found in plant based foods such as beans, lentils, fruits, vegetables, whole grains, etc One of the major functions of fiber is to add bulk to stools and promote regular bowel movements Often, a low fiber diet is then used to reduce stool bulk and output. The diet is used for various medical conditions, such as in ulcerative colitis, during the recovery period after intestinal surgery or to reduce risk of an intestinal obstruction if there is a stricture (narrowing) present in the intestinal tract. A low fiber diet is usually about 10-15 grams of fiber per day Although fiber is not found in meats, poultry, fish, eggs and dairy, it may help to promote overall tolerance to foods if you: Avoid tough and fibrous cuts of meats, poultry, etc. Eat cuts that are soft and tender. Avoid large amounts of dairy if dairy is found to be bothersome (examples- cheese sauces, cream sauces etc). Eat dairy then in small amounts and spread out dairy between all the meals. Avoid any meat, poultry, fish, eggs and dairy made with high fiber ingredients (dried fruits, nuts, seeds, etc) 1 Tips for a Low Fiber Diet: There is always more than one way to eat a specific food. It will help to peel, cook and/or blend your foods with fiber to optimize tolerance and enjoy variety in the diet. Eat small frequent meals throughout the day (4-6 meals/day). -

Cassava in South America and the Caribbean*
Color profile: Disabled Composite Default screen Chapter 2 Cassava in South America and the Caribbean* Guy Henry1 and Clair Hershey2 1CIRAD-Amis, Rua Paulo Castro P. Nogueira 600, Campinas-SP 13092–400, Brazil; 22019 Locust Grove Road, Manheim, PA 17545, USA Origin and Distribution of Cassava homelands. This history has not only had a pro- in Latin America found influence on the current status of the crop, but also on its potential for further development. Cassava and all its wild relatives have their Cassava has numerous traits that confer genetic origins in Latin America (the term Latin comparative advantages in marginal environ- America is used herein for the entire cassava- ments, where farmers often lack the resources to growing region of the New World). The crop was improve the income-generating capacity of their vital to the development of lowland tropical cul- land through purchased inputs. The species tol- tures throughout the New World. The Carib and erates acid soils, periodic and extended drought, Arawak Indians of the Caribbean and northern and defoliation by pests. It is highly compatible South America were probably some of the earli- with many types of intercrops and flexible as to est cultivators of cassava, and many of their cus- time of harvest. Furthermore, the crop serves a toms of cultivation and processing remain virtu- wide variety of food, feed and industrial purposes. ally intact today, in that region, and throughout These traits have combined to make cassava a the Amazon basin. Every tropical country of the significant sustaining force, benefiting the poor region produces cassava, but its cultivation is in the tropics.