Deirochelys Reticularia (Latreille 1801) – Chicken Turtle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Year of the Turtle News No
Year of the Turtle News No. 1 January 2011 Basking in the Wonder of Turtles www.YearoftheTurtle.org Welcome to 2011, the Wood Turtle, J.D. Kleopfer Bog Turtle, J.D. Willson Year of the Turtle! Turtle conservation groups in partnership with PARC have designated 2011 as the Year of the Turtle. The Chinese calendar declares 2011 as the Year of the Rabbit, and we are all familiar with the story of the “Tortoise and the Hare”. Today, there Raising Awareness for Turtle State of the Turtle Conservation is in fact a race in progress—a race to extinction, and turtles, unfortunately, Trouble for Turtles Our Natural Heritage of Turtles are emerging in the lead, ahead The fossil record shows us that While turtles (which include of birds, mammals, and even turtles, as we know them today, have tortoises) occur in fresh water, salt amphibians. The majority of turtle been on our planet since the Triassic water, and on land, their shells make threats are human-caused, which also Period, over 220 million years ago. them some of the most distinctive means that we can work together to Although they have persisted through animals on Earth. Turtles are so address turtle conservation issues many tumultuous periods of Earth’s unique that some scientists argue that and to help ensure the continued history, from glaciations to continental they should be in their own Class of survival of these important animals. shifts, they are now at the top of the vertebrates, Chelonia, separate from Throughout the year we will be raising list of species disappearing from the reptiles (such as lizards and snakes) awareness of the issues surrounding planet: 47.6% of turtle species are and other four-legged creatures. -

AN INTRODUCTION to Texas Turtles
TEXAS PARKS AND WILDLIFE AN INTRODUCTION TO Texas Turtles Mark Klym An Introduction to Texas Turtles Turtle, tortoise or terrapin? Many people get confused by these terms, often using them interchangeably. Texas has a single species of tortoise, the Texas tortoise (Gopherus berlanderi) and a single species of terrapin, the diamondback terrapin (Malaclemys terrapin). All of the remaining 28 species of the order Testudines found in Texas are called “turtles,” although some like the box turtles (Terrapene spp.) are highly terrestrial others are found only in marine (saltwater) settings. In some countries such as Great Britain or Australia, these terms are very specific and relate to the habit or habitat of the animal; in North America they are denoted using these definitions. Turtle: an aquatic or semi-aquatic animal with webbed feet. Tortoise: a terrestrial animal with clubbed feet, domed shell and generally inhabiting warmer regions. Whatever we call them, these animals are a unique tie to a period of earth’s history all but lost in the living world. Turtles are some of the oldest reptilian species on the earth, virtually unchanged in 200 million years or more! These slow-moving, tooth less, egg-laying creatures date back to the dinosaurs and still retain traits they used An Introduction to Texas Turtles | 1 to survive then. Although many turtles spend most of their lives in water, they are air-breathing animals and must come to the surface to breathe. If they spend all this time in water, why do we see them on logs, rocks and the shoreline so often? Unlike birds and mammals, turtles are ectothermic, or cold- blooded, meaning they rely on the temperature around them to regulate their body temperature. -

N.C. Turtles Checklist
Checklist of Turtles Historically Encountered In Coastal North Carolina by John Hairr, Keith Rittmaster and Ben Wunderly North Carolina Maritime Museums Compiled June 1, 2016 Suborder Family Common Name Scientific Name Conservation Status Testudines Cheloniidae loggerhead Caretta caretta Threatened green turtle Chelonia mydas Threatened hawksbill Eretmochelys imbricata Endangered Kemp’s ridley Lepidochelys kempii Endangered Dermochelyidae leatherback Dermochelys coriacea Endangered Chelydridae common snapping turtle Chelydra serpentina Emydidae eastern painted turtle Chrysemys picta spotted turtle Clemmys guttata eastern chicken turtle Deirochelys reticularia diamondback terrapin Malaclemys terrapin Special concern river cooter Pseudemys concinna redbelly turtle Pseudemys rubriventris eastern box turtle Terrapene carolina yellowbelly slider Trachemys scripta Kinosternidae striped mud turtle Kinosternon baurii eastern mud turtle Kinosternon subrubrum common musk turtle Sternotherus odoratus Trionychidae spiny softshell Apalone spinifera Special concern NOTE: This checklist was compiled and updated from several sources, both in the scientific and popular literature. For scientific names, we have relied on: Turtle Taxonomy Working Group [van Dijk, P.P., Iverson, J.B., Rhodin, A.G.J., Shaffer, H.B., and Bour, R.]. 2014. Turtles of the world, 7th edition: annotated checklist of taxonomy, synonymy, distribution with maps, and conservation status. In: Rhodin, A.G.J., Pritchard, P.C.H., van Dijk, P.P., Saumure, R.A., Buhlmann, K.A., Iverson, J.B., and Mittermeier, R.A. (Eds.). Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs 5(7):000.329–479, doi:10.3854/crm.5.000.checklist.v7.2014; The IUCN Red List of Threatened Species. -

In AR, FL, GA, IA, KY, LA, MO, OH, OK, SC, TN, and TX): Species in Red = Depleted to the Point They May Warrant Federal Endangered Species Act Listing
Southern and Midwestern Turtle Species Affected by Commercial Harvest (in AR, FL, GA, IA, KY, LA, MO, OH, OK, SC, TN, and TX): species in red = depleted to the point they may warrant federal Endangered Species Act listing Common snapping turtle (Chelydra serpentina) – AR, GA, IA, KY, MO, OH, OK, SC, TX Florida common snapping turtle (Chelydra serpentina osceola) - FL Southern painted turtle (Chrysemys dorsalis) – AR Western painted turtle (Chrysemys picta) – IA, MO, OH, OK Spotted turtle (Clemmys gutatta) - FL, GA, OH Florida chicken turtle (Deirochelys reticularia chrysea) – FL Western chicken turtle (Deirochelys reticularia miaria) – AR, FL, GA, KY, MO, OK, TN, TX Barbour’s map turtle (Graptemys barbouri) - FL, GA Cagle’s map turtle (Graptemys caglei) - TX Escambia map turtle (Graptemys ernsti) – FL Common map turtle (Graptemys geographica) – AR, GA, OH, OK Ouachita map turtle (Graptemys ouachitensis) – AR, GA, OH, OK, TX Sabine map turtle (Graptemys ouachitensis sabinensis) – TX False map turtle (Graptemys pseudogeographica) – MO, OK, TX Mississippi map turtle (Graptemys pseuogeographica kohnii) – AR, TX Alabama map turtle (Graptemys pulchra) – GA Texas map turtle (Graptemys versa) - TX Striped mud turtle (Kinosternon baurii) – FL, GA, SC Yellow mud turtle (Kinosternon flavescens) – OK, TX Common mud turtle (Kinosternon subrubrum) – AR, FL, GA, OK, TX Alligator snapping turtle (Macrochelys temminckii) – AR, FL, GA, LA, MO, TX Diamond-back terrapin (Malaclemys terrapin) – FL, GA, LA, SC, TX River cooter (Pseudemys concinna) – AR, FL, -

Ecolo Arkan Ogy of the W Nsas Valley D Western C Y: Develop Dr. Stephen Un Fin Chicken Tu Ment of Su and Sec Ju N Di
Final Report: Ecology of the Western Chicken Turtle (Deirochelys reticularia miaria) in the Arkansas Valley: Development of Survey and Monitoring Protocols for a Rare and Secretive Species June 2009 Dr. Stephen Dinkelacker and Nathanael Hilzinger Department of Biology University of Central Arkansas Conway, AR 72035 Introduction: Because of its scarcity and poorly understood biology and ecology, the Western Chicken Turtle (Deirochelys reticularia miaria) has been designated as an S3 species (i.e., rare to uncommon) with fewer than 25 locality records in Arkansas. At this time, development of a conservation and management plan is inconceivable due to a lack of baseline ecological data. This information is critical for the development of suitable conservation strategies and to define appropriate distribution and abundance survey protocols for this rare species. Virtually nothing is known about the Western Chicken Turtle (D. r. miaria) in Arkansas, except that they occur in the state. In fact, little is known about the ecology of the three Chicken Turtle subspecies. Few studies have examined the ecology of Chicken Turtles since 1969, and those were conducted on the eastern subspecies (D. r. reticularia) in Virginia and South Carolina. Most accounts of Chicken Turtles that appear in general herpetological surveys and reference books lack or provide insufficient ecological data. The limited ecological and biological data that are available for Chicken Turtles suggest they use a variety of habitats, including terrestrial habitats, during their annual cycle. Chicken Turtles may have bimodal nesting seasons, with the first season occurring in early spring and the second in late summer/early fall. Preliminary data from a local population suggests that female Western Chicken Turtles are gravid in mid‐June, potentially using terrestrial habitats for most of the year, and occupying seasonal wetlands during late spring and early summer. -

North Louisiana Refuges Complex: Freshwater Turtle Inventory
NORTH LOUISIANA REFUGES COMPLEX: FRESHWATER TURTLE INVENTORY USFWS Award No: F17PX01556 John L. Carr, Aaron C. Johnson & J. Benjamin Grizzle November 2020 ACKNOWLEDGMENTS We thank the U.S. Fish and Wildlife Service Region 4 Inventory and Monitoring Branch for USFWS Award No. F17PX01556, “Freshwater Turtle Inventory of the North Louisiana Refuges Complex”. In addition, this report incorporates data from other, complementary projects that were funded by a variety of sources. This has been done in order to provide a more fulsome picture of knowledge on the turtle fauna of the refuges within the Complex. These other projects were funded in part by the Louisiana Department of Wildlife and Fisheries and the U.S. Fish and Wildlife Service, Division of Federal Aid, through the State Wildlife Grants Program (series of projects targeting the Alligator Snapping Turtle and map turtles). Other sources include data collected for grant activities funded by USGS-BRD (Cooperative Agreement No. 99CRAG0017), funding from the Louisiana Wildlife and Fisheries Foundation beginning in 1998, funding directly from LDWF (2000-2002), The Nature Conservancy (contract #LAFO_022309), Friends of Black Bayou, the Turtle Research Fund of the University of Louisiana at Monroe Foundation, and the Kitty DeGree Professorship in Biology (2011-2017). U.S. Fish and Wildlife personnel helped facilitate our work by granting access to all parts of the refuges at various times, and providing a series of Special Use Permits over 20+ years. We acknowledge Lee Fulton, Joe McGowan, Brett Hortman, Kelby Ouchley, and George Chandler for facilitating our work on refuges over the years; in particular, we thank Gypsy Hanks for long-sustained support and cooperation, especially during the course of the current project. -

Estuaries to Edwards
Glenn Hegar Texas Comptroller of Public Accounts Estuaries to Edwards Aquatic Species Research with the Texas Comptroller's Natural Resources Program Glenn Hegar Texas Comptroller of Public Accounts Outline 1. The Comptroller’s role in endangered species issues 2. The Natural Resources programs process 3. Ongoing projects Glenn Hegar Texas Comptroller of Public Accounts Natural Resources Program The program collaborates with communities & stakeholders to identify knowledge gaps and support ecological research to contribute to the ESA listing process and long-term conservation strategies. Natural Resources Homepage Glenn Hegar Texas Comptroller of Public Accounts Identify needs… Glenn Hegar Texas Comptroller of Public Accounts Endangered Species Act 101 The U.S. Fish and Wildlife Service implements the Endangered Species Act of 1975. • Threatened and Endangered Species list • Recovery planning • Voluntary conservation agreements Monitor the USFWS federal register homepage for listing decisions, recovery plans, and more. Glenn Hegar Texas Comptroller of Public Accounts Poll Question #1 Which of the following Texas species is NOT federally endangered? A. Texas Hornshell B. Alligator Snapping Turtle C. Comanche Springs Pupfish D. Texas Blind Salamander Glenn Hegar Texas Comptroller of Public Accounts Endangered Species Act 101 Petition 90-day Listing finding decision 90 days 12 months Glenn Hegar Texas Comptroller of Public Accounts Endangered Species Act 101 Petition 90-day SSA Listing finding process decision 90 days 12 months Glenn Hegar -

Proposed Amendment to 21CFR124021
Richard Fife 8195 S. Valley Vista Drive Hereford, AZ 85615 December 07, 2015 Division of Dockets Management Food and Drug Administration 5630 Fishers Lane, rm. 1061 Rockville, MD 20852 Reference: Docket Number FDA-2013-S-0610 Proposed Amendment to Code of Federal Regulations Title 21, Volume 8 Revised as of April 1, 2015 21CFR Sec.1240.62 Dear Dr. Stephen Ostroff, M.D., Acting Commissioner: Per discussion with the Division of Dockets Management staff on November 10, 2015 Environmental and Economic impact statements are not required for petitions submitted under 21CFR Sec.1240.62 CITIZEN PETITION December 07, 2015 ACTION REQUESTED: I propose an amendment to 21CFR Sec.1240.62 (see exhibit 1) as allowed by Section (d) Petitions as follows: Amend section (c) Exceptions. The provisions of this section are not applicable to: By adding the following two (2) exceptions: (5) The sale, holding for sale, and distribution of live turtles and viable turtle eggs, which are sold for a retail value of $75 or more (not to include any additional turtle related apparatuses, supplies, cages, food, or other turtle related paraphernalia). This dollar amount should be reviewed every 5 years or more often, as deemed necessary by the department in order to make adjustments for inflation using the US Department of Labor, Bureau of labor Statistics, Consumer Price Index. (6) The sale, holding for sale, and distribution of live turtles and viable turtle eggs, which are listed by the International Union for Conservation of Nature and Natural Resources (IUCN) Red List as Extinct In Wild, Critically Endangered, Endangered, or Vulnerable (IUCN threatened categorizes). -

A Systematic Review of the Turtle Family Emydidae
67 (1): 1 – 122 © Senckenberg Gesellschaft für Naturforschung, 2017. 30.6.2017 A Systematic Review of the Turtle Family Emydidae Michael E. Seidel1 & Carl H. Ernst 2 1 4430 Richmond Park Drive East, Jacksonville, FL, 32224, USA and Department of Biological Sciences, Marshall University, Huntington, WV, USA; [email protected] — 2 Division of Amphibians and Reptiles, mrc 162, Smithsonian Institution, P.O. Box 37012, Washington, D.C. 200137012, USA; [email protected] Accepted 19.ix.2016. Published online at www.senckenberg.de / vertebrate-zoology on 27.vi.2016. Abstract Family Emydidae is a large and diverse group of turtles comprised of 50 – 60 extant species. After a long history of taxonomic revision, the family is presently recognized as a monophyletic group defined by unique skeletal and molecular character states. Emydids are believed to have originated in the Eocene, 42 – 56 million years ago. They are mostly native to North America, but one genus, Trachemys, occurs in South America and a second, Emys, ranges over parts of Europe, western Asia, and northern Africa. Some of the species are threatened and their future survival depends in part on understanding their systematic relationships and habitat requirements. The present treatise provides a synthesis and update of studies which define diversity and classification of the Emydidae. A review of family nomenclature indicates that RAFINESQUE, 1815 should be credited for the family name Emydidae. Early taxonomic studies of these turtles were based primarily on morphological data, including some fossil material. More recent work has relied heavily on phylogenetic analyses using molecular data, mostly DNA. The bulk of current evidence supports two major lineages: the subfamily Emydinae which has mostly semi-terrestrial forms ( genera Actinemys, Clemmys, Emydoidea, Emys, Glyptemys, Terrapene) and the more aquatic subfamily Deirochelyinae ( genera Chrysemys, Deirochelys, Graptemys, Malaclemys, Pseudemys, Trachemys). -

Heinrich and Walsh (2019)
THE BIG TURTLE YEAR LOOKING FOR WILD TURTLES IN WILD PLACES By George L. Heinrich and Timothy J. Walsh e like looking for wild turtles in wild We both liked turtles as kids, but now, many places. From the time George was in years later, we understand the important ecological Welementary school catching Wood roles they play. Some turtle species serve as indica- Turtles in southwestern Connecticut and Tim tors of environmental health, while others are clas- found his first pebble-sized Striped Mud Turtle sified as keystone species (those that play a vital at age 10 in a south Florida stream, we have both ecological role in a given habitat), umbrella spe- marveled at being in nature and searching for these cies (those whose conservation benefits the larger fascinating reptiles. As children, little did we know ecological community), or flagship species (iconic that our time spent exploring our neighborhood symbols of habitat conservation efforts). Perhaps woods would lead to rewarding careers in wildlife one of the best examples of the ecological signifi- conservation. Now we are both officers with the cance of turtles is the Gopher Tortoise, an imper- Florida Turtle Conservation Trust, an organization iled species that occurs in a six-state range within working to conserve Florida’s rich turtle diversity. the Southeastern Coastal Plain of the United States. In 2017 we created the opportunity of a Appropriately, the Gopher Tortoise was the first lifetime with the Trust’s conservation education species we found on day one of The Big Turtle Year. initiative: The Big Turtle Year, an ambitious plan to travel across the United States and back again SoutheaSt Regional highlightS trying to find as many species as we could. -
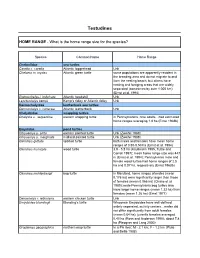
Home Range Testudines
Testudines HOME RANGE - What is the home range size for the species? Species Common Name Home Range Cheloniidae sea turtles Caretta c. caretta Atlantic loggerhead Unk Chelonia m. mydas Atlantic green turtle some populations are apparently resident in the breeding area and do not migrate to and from the nesting beach, but others have nesting and foraging areas that are widely separated (sometimes by over 1,000 km) (Ernst et al. 1994) Eretmochelys i. imbricata Atlantic hawksbill Unk Lepidochelys kempii Kemp’s ridley or Atlantic ridley Unk Dermochelyidae leatherback sea turtles Dermochelys c. coriacea Atlantic leatherback Unk Chelydridae snapping turtles Chelydra s. serpentina eastern snapping turtle in Pennsylvanina, nine adults…had estimated home ranges averaging 1.8 ha (Ernst 1968b) Emydidae pond turtles Chrysemys p. picta eastern painted turtle Unk (Zweifel 1989) Chrysemys p. marginata midland painted turtle Unk (Zweifel 1989) Clemmys guttata spotted turtle both males and females have mean home ranges of 0.50-0.53 ha (Ernst et al. 1994) Clemmys insculpta wood turtle 3.9 - 5.8 ha (Kaufmann 1995, Tuttle and Carroll 1997); mean home range size was 447 m (Ernst et al. 1994); Pennsylvania male and female wood turtles had home ranges of 2.5 ha and 0.07 ha, respectively (Ernst 1968b) Clemmys muhlenbergii bog turtle in Maryland, home ranges of males (mean 0.176 ha) were significantly larger than those of females (mean 0.066 ha) (Chase et al. 1989); male Pennsylvania bog turtles also have larger home ranges (mean 1.33 ha) than females (mean 1.26 ha) (Ernst 1977) Deirochelys r. -

Water Turtles
WATER TURTLES Range Bog Turtle Bog Turtle • North America’s smallest turtle (3” to 4.5”); NC’s rarest • Have noticeable bright orange, yellow or red blotch on each side of face • Live in isolated spring-fed fen, sphagnum bogs, marshy meadows and wet pastures • Eat beetles, insect larvae, snails, seeds and millipedes • Females mature from 5-8 years and mate from May to Yellow-bellied Slider June Range • Deposit 2-6 eggs from June to July which hatch after 42 -56 days of incubation • Placed on NC’s threatened list in 1989 ENDANGERED 1 Yellow-bellied Slider • Yellow patch on side of head – on females and juveniles • 5 to 8 inches • Underside of shell is yellow • Males smaller than females with longer, thicker tail and long fingernails • Juveniles eat more carnivorous diet Redbelly Turtle • Adults are omnivores – feeding = underwater • Males mature between 3-5 yrs • 5-7 year old females lay 4 to 23 eggs from May to July • Eggs incubate 2-2.5 months and hatch between July- September • Help control invertebrate and vegetation populations • Live in fresh water Range Red Belly Turtle • 10-15” in length • Like deep water • Eat both plants and animals-insect larvae, crayfish, worms and tadpoles • Active from May thru October Snapping Turtle • Females lay 10-12 eggs in June-July which hatch Range sometime in late summer. • Young sometimes winter over in the nest until spring • Like to bask in the sun 2 Snapping Turtle • 8-14 inches weighing from 10 to 50 lbs • Large head, small plastron, long tail & strong limbs • Males larger than females Range