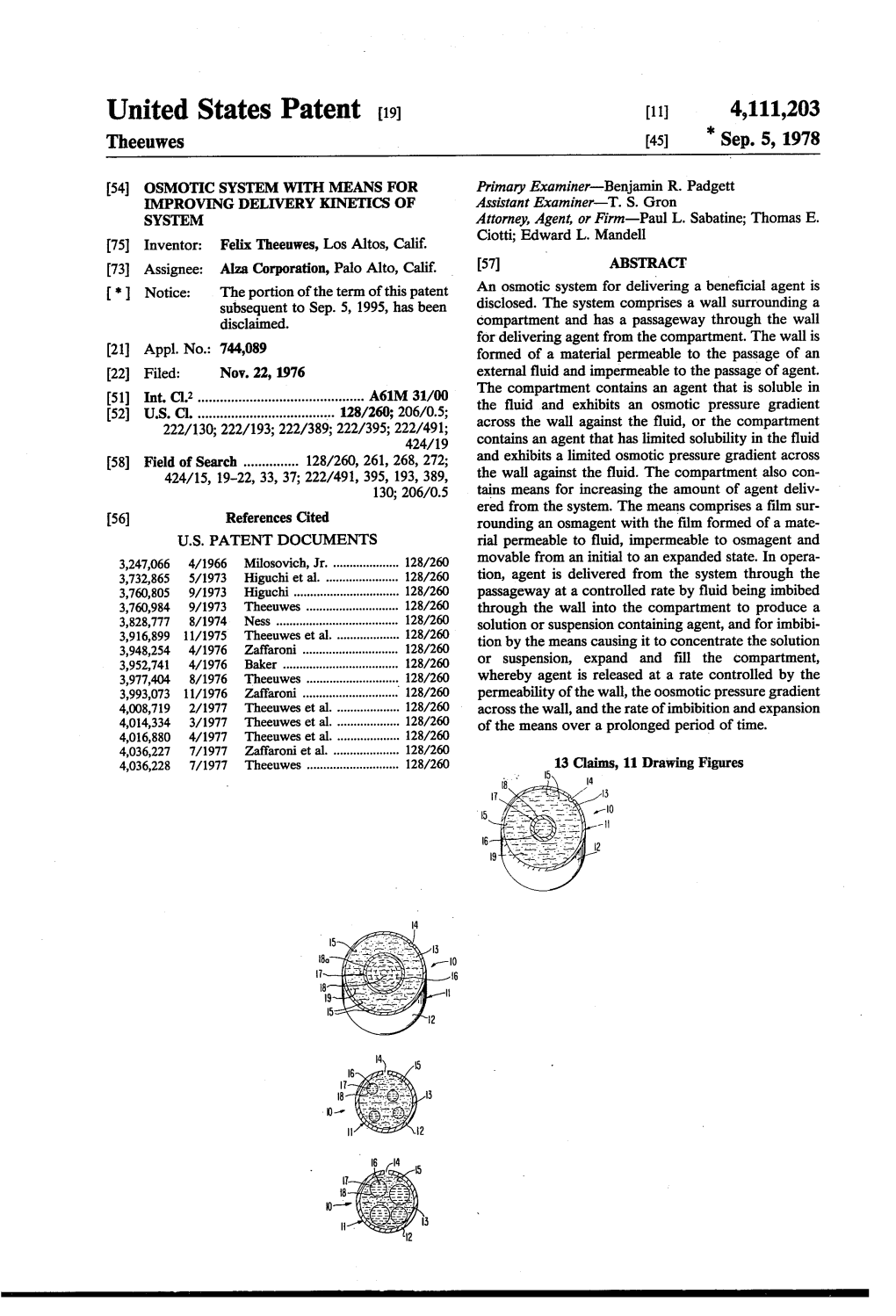
United States Patent (19) 11 4,111,203 Theeuwes (45) "Sep
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent Office
3,092,548 United States Patent Office Patented June 4, 1963 2 are the various side effects that occur in an appreciable 3,092,548 number of patients, the foremost of which are a blurring METHOD OF TREATING PEPTICULCERS WITH PANTOTHENECACD of vision, drowsiness and a general dry condition mani Albert G. Worton, Columbus, Ohio, assignor to The fested by a retarded salivation, reduction of perspiration Warren-Teed Products Company, Columbus, Ohio, a 5 and diminished urinary output. Other side effects which corporation of Ohio occur in some cases are glaucoma, stimulation of the cen No Drawing. Filed Oct. 27, 1960, Ser. No. 65,256 trol nervous system and in severe cases cardiac and respi 5 Claims. (Ci. 67-55) ratory collapse. These side effects increase to Some de gree with the increase in dosage. Despite the side effects This invention relates to preparation adapted for use O these compounds are fairly selective and highly effective in treating disorders of the gastro-intestinal tract, more in decreasing the volume and acidity of gastric secretion particularly in treating peptic ulcer, both of the duodenal and in reducing gastrointestinal motility. and gastric type, for hyperacidity, hypertropic gastritis, Understandably, the main problem in the past has been splenic flexure syndrome, biliary dyskinesia (postchole to provide a dosage of anticholinergic drug which will cystectomy syndrome) and hiatal hernia or other condi achieve the most beneficial results possible and yet mini tions where anticholinergic effect, either spasmolytic or mize the undesired side effects. One of the objects of antisecretory is indicated or where antiuicerogenic effect this invention is to provide novel compositions containing is indicated. -

Isopropamide Iodide
www.chemicalland21.com ISOPROPAMIDE IODIDE SYNONYMS (3-Carbamoyl-3,3-diphenylpropyl)diisopropylmethylammonium iodide; 2,2-Diphenyl-4- diisopropylaminobutyramide methiodide; 4-(Diisopropylamino)-2,2-diphenylbutyramide methiodide; gamma-(Aminocarbonyl)-N-methyl-N,N-bis(1-methylethyl)-gamma-phenylbenzenepropanaminium iodide; Iodure d'isopropamide; Ioduro de isopropamida; Isopropamide ioduro; Isopropamidi iodidum; Isoproponum iodide; PRODUCT IDENTIFICATION CAS RN 71-81-8 EINECS RN 200-766-8 FORMULA C23H33IN2O MOL WEIGHT 480.43 PHYSICAL AND CHEMICAL PROPERTIES PHYSICAL STATE white to off-white powder MELTING POINT 199 C BOILING POINT DENSITY SOLUBILITY IN WATER pH VAPOR DENSITY REFRACTIVE INDEX FLASH POINT GENERAL DESCRIPTION Isopropamide is a long-acting anticholinergic and antimuscarinic drug of quaternary ammonium structure. It is used in the form of the iodide, (also bromide or chloride) to treat peptic ulcer and to suppress gastric secretion other gastrointestinal disorders. Brands of Isopropamide drugs: Darbid Dipramide Isamide Marygin-M Piaccamide Priamide Priazimide Sanulcin Tyrimide Quaternary ammonium anticholinergics (Synthetic) ATC Code Product CAS RN. A03AB01 Benzilonium bromide 1050-48-2 A03AB02 Glycopyrrolate 596-51-0 A03AB03 Oxyphenonium 14214-84-7 A03AB04 Penthienate 22064-27-3 A03AB05 Propantheline 50-34-0 A03AB06 Otilonium bromide 26095-59-0 A03AB07 Methantheline 5818-17-7 Please mail us if you want to sell your product or need to buy some products) www.chemicalland21.com ISOPROPAMIDE IODIDE A03AB08 Tridihexethyl 60-49-1 A03AB09 Isopropamide 7492-32-2 A03AB10 Hexocyclium 6004-98-4 A03AB11 Poldine 596-50-9 A03AB12 Mepenzolic acid 25990-43-6 A03AB13 Bevonium 33371-53-8 A03AB14 Pipenzolate 13473-38-6 A03AB15 Diphemanil methylsulfate 62-97-5 A03AB16 (2-Benzhydryloxyethyl)diethyl-methylammonium iodide A03AB17 Tiemonium iodide 144-12-7 A03AB18 Prifinium bromide 4630-95-9 A03AB19 Timepidium bromide 35035-05-3 A03AB21 Fenpiverinium bromide 125-60-0 03AB53 Oxyphenonium, combinations STABILITY AND REACTIVITY STABILITY Stable under normal conditions. -

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

The In¯Uence of Medication on Erectile Function
International Journal of Impotence Research (1997) 9, 17±26 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 The in¯uence of medication on erectile function W Meinhardt1, RF Kropman2, P Vermeij3, AAB Lycklama aÁ Nijeholt4 and J Zwartendijk4 1Department of Urology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; 2Department of Urology, Leyenburg Hospital, Leyweg 275, 2545 CH The Hague, The Netherlands; 3Pharmacy; and 4Department of Urology, Leiden University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands Keywords: impotence; side-effect; antipsychotic; antihypertensive; physiology; erectile function Introduction stopped their antihypertensive treatment over a ®ve year period, because of side-effects on sexual function.5 In the drug registration procedures sexual Several physiological mechanisms are involved in function is not a major issue. This means that erectile function. A negative in¯uence of prescrip- knowledge of the problem is mainly dependent on tion-drugs on these mechanisms will not always case reports and the lists from side effect registries.6±8 come to the attention of the clinician, whereas a Another way of looking at the problem is drug causing priapism will rarely escape the atten- combining available data on mechanisms of action tion. of drugs with the knowledge of the physiological When erectile function is in¯uenced in a negative mechanisms involved in erectile function. The way compensation may occur. For example, age- advantage of this approach is that remedies may related penile sensory disorders may be compen- evolve from it. sated for by extra stimulation.1 Diminished in¯ux of In this paper we will discuss the subject in the blood will lead to a slower onset of the erection, but following order: may be accepted. -

Poisons and Narcotic Drugs (Amendment) Ordinance 1988
AUSTRALIAN CAPITAL TERRITORY Poisons and Narcotic Drugs (Amendment) Ordinance 1988 No. 96 of 1988 I, THE GOVERNOR-GENERAL of the Commonwealth of Australia, acting with the advice of the Federal Executive Council, hereby make the following Ordinance under the Seat of Government (Administration) Act 1910. Dated 15 December 1988 N. M. STEPHEN Governor-General By His Excellency’s Command, CLYDE HOLDING Minister of State for the Arts and Territories An Ordinance to amend the Poisons and Narcotic Drugs Ordinance 1978 Short title 1. This Ordinance may be cited as the Poisons and Narcotic Drugs (Amendment) Ordinance 1988.1 Commencement 2. This Ordinance commences on such date as is fixed by the Minister by notice in the Gazette. Principal Ordinance 3. In this Ordinance, “Principal Ordinance” means the Poisons and Narcotic Drugs Ordinance 1978.2 (Ord. 79/88)—Cat. No. Authorised by the ACT Parliamentary Counsel—also accessible at www.legislation.act.gov.au 2 Poisons and Narcotic Drugs (Amendment) No. 96, 1988 Substances to which Division applies 4. Section 27B of the Principal Ordinance is amended by adding at the end the following paragraphs: “; (f) follicle stimulating hormone; (g) luteinising hormone; (h) thalidomide.”. Grant of authorisation 5. Section 27E of the Principal Ordinance is amended— (a) by omitting from paragraph (1) (a) “or cyclofenil” and substituting “, cyclofenil, follicle stimulating hormone or luteinising hormone”; (b) by omitting from paragraph (1) (b) “and”; and (c) by adding at the end of subsection (1) the following word and paragraph: “; and (d) in the case of an application that relates to thalidomide— the applicant is a specialist physician with no less than 5 years’ experience in the treatment of erythema nodosum leprosum.”. -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

Development and Validation of Spectrophotometric Method for Simultaneous Determination of Isopropamide and Trifluoperazine in Tablet Dosage Form
Development and Validation of Spectrophotometric Method for Simultaneous Determination of Isopropamide and Trifluoperazine in Tablet Dosage Form Masimukku Siva Kishore1,2, Chintala Rambabu1 1Department of Chemistry, Acharya Nagarjuna University, Guntur, Andhra Pradesh, India, 2Department of Chemistry, KBN PG College, Vijayawada, Andhra Pradesh, India. Abstract ORIGINAL ARTICLE ORIGINAL Aim: The aim of this study is to develop simple, sensitive, rapid, accurate, precise and economical spectrophotometric method based on simultaneous equation method for the simultaneous estimation of isopropamide and trifluoperazine in combined tablet dosage form. Materials and Methods: The method is based on the simultaneous equation and first-order derivative method for analysis of both the drugs using methanol:water in the ratio of 7:3 (v/v) as solvent. Isopropamide has absorbance maxima at 248.5 nm and trifluoperazine has absorbance maxima at 227.0 nm in methanol. Results and Discussion: The linearity was obtained in the concentration range of 5-30 µg/mL for isopropamide and 2-12 µg/mL for trifluoperazine in both the methods. The concentration of drugs was determined by using simultaneous equation method. The result of analysis has been validated statistically and by recovery studies. The results of mean recovery and other validation parameters were found within the acceptable limits. Both methods were applied to estimate the marketed formulation and found good recovery of the drug. The methods were found to be simple, sensitive, accurate and precise and were applicable for simultaneous determination of isopropamide and trifluoperazine in pharmaceutical dosage form. Key words: Isopropamide, method development and validation, trifluoperazine, ultraviolet spectrophotometer. INTRODUCTION spasm.[10] The drug works by inhibiting parasympathetic nerve impulses by selectively blocking the binding of the rifluoperazine [Figure 1a] is phenothiazine neurotransmitter acetylcholine to its receptor in nerve chemical class drug used for short- cells. -

Wo 2010/075090 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 1 July 2010 (01.07.2010) WO 2010/075090 A2 (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C07D 409/14 (2006.01) A61K 31/7028 (2006.01) kind of national protection available): AE, AG, AL, AM, C07D 409/12 (2006.01) A61P 11/06 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US2009/068073 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 15 December 2009 (15.12.2009) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, (26) Publication Language: English TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/122,478 15 December 2008 (15.12.2008) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (71) Applicant (for all designated States except US): AUS- ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, PEX PHARMACEUTICALS, INC. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Federal Register/Vol. 77, No. 115/Thursday, June 14, 2012
Federal Register / Vol. 77, No. 115 / Thursday, June 14, 2012 / Notices 35691 TABLE 1—LIST OF SAFETY AND EFFECTIVENESS SUMMARIES FOR APPROVED PMAS MADE AVAILABLE FROM JANUARY 1, 2012, THROUGH MARCH 31, 2012—Continued PMA No., Docket No. Applicant Trade name Approval date P060008.S046, FDA–2012–M–0210 ... Boston Scientific Corp ......................... TAXUS Liberte´ Paclitaxel-Eluting Cor- February 22, 2012. onary Stent System (Monorail and Over-The-Wire Delivery Systems). P030025.S086, FDA–2012–M–0209 ... Boston Scientific Corp ......................... TAXUS Express2 Paclitaxel-Eluting February 22, 2012. Coronary Stent System (Monorail and Over-The-Wire Delivery Sys- tems). P110023, FDA–2012–M–0221 ............ ev3, Inc ................................................ Everflex Self-Expanding Peripheral March 7, 2012. Stent System (Everflex). P070004, FDA–2012–M–0250............ Sientra, Inc.......................................... SIENTRA Silicone Gel Breast Im- March 9, 2012. plants. II. Electronic Access LOCATION: The meeting will be held at submissions. In the process of Persons with access to the Internet the FDA White Oak Campus, 10903 considering these changes, FDA has may obtain the documents at http:// New Hampshire Ave., Bldg. 31 previously made available for comment www.fda.gov/MedicalDevices/ Conference Center, Great Room 1503, versions of documents that support ProductsandMedicalProcedures/ Silver Spring, MD 20993. The following making regulatory submissions in DeviceApprovalsandClearances/ link contains public meeting attendee electronic format using the (eCTD) information as well as frequently asked PMAApprovals/default.htm and http:// specifications. These draft documents questions and answers regarding public www.fda.gov/MedicalDevices/ represented FDA’s major updates to meetings at White Oak: http:// ProductsandMedicalProcedures/ Module 1 of the eCTD based on www.fda.gov/AboutFDA/ DeviceApprovalsandClearances/ previous comments. -

NVA237 / Glycopyrronium Bromide Multinational, Multi-Database Drug
Quantitative Safety & Epidemiology NVA237 / Glycopyrronium bromide Non-interventional Final Study Report NVA237A2401T Multinational, multi-database drug utilization study of inhaled NVA237 in Europe Author Document Status Final Date of final version 28 April 2016 of the study report EU PAS register ENCEPP/SDPP/4845 number Property of Novartis Confidential May not be used, divulged, published or otherwise disclosed without the consent of Novartis NIS Report Template Version 2.0 August-13-2014 Novartis Confidential Page 2 Non-interventional study report NVA237A/Seebri® Breezhaler®/CNVA237A2401T PASS information Title Multinational, multi-database drug utilization study of inhaled NVA237 in Europe –Final Study Report Version identifier of the Version 1.0 final study report Date of last version of 28 April 2016 the final study report EU PAS register number ENCEPP/SDPP/4845 Active substance Glycopyrronium bromide (R03BB06) Medicinal product Seebri®Breezhaler® / Tovanor®Breezhaler® / Enurev®Breezhaler® Product reference NVA237 Procedure number SeebriBreezhaler: EMEA/H/C/0002430 TovanorBreezhaler: EMEA/H/C/0002690 EnurevBreezhaler: EMEA/H/C0002691 Marketing authorization Novartis Europharm Ltd holder Frimley Business Park Camberley GU16 7SR United Kingdom Joint PASS No Research question and In the context of the NVA237 marketing authorization objectives application, the Committee for Medicinal Products for Human Use (CHMP) recommended conditions for marketing authorization and product information and suggested to conduct a post-authorization -

The Pharmacology of Paediatric Incontinence
BJU International (2000), 86, 581±589 The pharmacology of paediatric incontinence P.B. HOEBEKE* and J. VANDE WALLE² *Departments of Paediatric Urology and ²Paediatric Nephrology, Ghant University Hospital, Belgium Introduction ' the accommodation of increasing volumes of urine The clinical uropharmacology of the lower urinary tract at a low intravesical pressure and with appropriate is based on an appreciation of the innervation and sensation receptor content of the bladder and its related anatomical ' a bladder outlet that is closed and remains so during structures. The anatomy, neuroanatomy and neuro- increases in intra-abdominal pressures physiology of the bladder is reviewed. Classes of drugs are ' and absence of involuntary bladder contractions [2]. discussed in relation to the possible functional targets of pharmacological intervention and ®nally some speci®c In children the development of continence and applications in paediatric voiding dysfunction are voluntary voiding involves maturation of the nervous discussed. system and behavioural learning. Toilet training mainly depends on the cognitive perception of the maturing urinary tract. This implies a high sensibility for the Pharmacological interactions development of dysfunctions [3]. The storage and A short review of anatomy, neuroanatomy and neuro- evacuation of urine are controlled by two functional physiology is essential to understand pharmacological units in the lower urinary tract, i.e. the reservoir (the interactions. These interactions with effects on bladder bladder) and an outlet (consisting of the bladder neck, function can occur on different levels, i.e. on bladder urethra and striated muscles of the pelvic ¯oor). In the mucosa, bladder smooth muscle or bladder outlet striated bladder, two regions (Fig.