Effects of Internal Loading on Algal Biomass in Lake Allatoona, a Southeastern Piedmont Impoundment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cobb County, Georgia and Incorporated Areas
VOLUME 1 OF 4 Cobb County COBB COUNTY, GEORGIA AND INCORPORATED AREAS COMMUNITY NAME COMMUNITY NUMBER ACWORTH, CITY OF 130053 AUSTELL, CITY OF 130054 COBB COUNTY 130052 (UNINCORPORATED AREAS) KENNESAW, CITY OF 130055 MARIETTA, CITY OF 130226 POWDER SPRINGS, CITY OF 130056 SMYRNA, CITY OF 130057 REVISED: MARCH 4, 2013 FLOOD INSURANCE STUDY NUMBER 13067CV001D NOTICE TO FLOOD INSURANCE STUDY USERS Communities participating in the National Flood Insurance Program have established repositories of flood hazard data for floodplain management and flood insurance purposes. This Flood Insurance Study (FIS) report may not contain all data available within the Community Map Repository. Please contact the Community Map Repository for any additional data. The Federal Emergency Management Agency (FEMA) may revise and republish part or all of this FIS report at any time. In addition, FEMA may revise part of this FIS report by the Letter of Map Revision process, which does not involve republication or redistribution of the FIS report. Therefore, users should consult with community officials and check the Community Map Repository to obtain the most current FIS report components. Initial Countywide FIS Effective Date: August 18, 1992 Revised Countywide FIS Effective Date: December 16, 2008 Revised Countywide FIS Effective Date: March 4, 2013 TABLE OF CONTENTS Page 1.0 INTRODUCTION 1 1.1 Purpose of Study 1 1.2 Authority and Acknowledgments 1 1.3 Coordination 3 2.0 AREA STUDIED 5 2.1 Scope of Study 5 2.2 Community Description 10 2.3 Principal Flood Problems -

Upper Apalachicola-Chattahoochee
Georgia: Upper Apalachicola- Case Study Chattahoochee-Flint River Basin Water Resource Strategies and Information Needs in Response to Extreme Weather/Climate Events ACF Basin The Story in Brief Communities in the Apalachicola-Chattahoochee-Flint River Basin (ACF) in Georgia, including Gwinnett County and the city of Atlanta, faced four consecutive extreme weather events: drought of 2007-08, floods of Sep- tember and winter 2009, and drought of 2011-12. These events cost taxpayers millions of dollars in damaged infrastructure, homes, and businesses and threatened water supply for ecological, agricultural, energy, and urban water users. Water utilities were faced with ensuring reliable service during and after these events. Drought of 2007-2008 and 2012 Impacts Northern Georgia saw record-low precipitation in 2007. By late spring 2008, Lake Lanier, the state’s major water supply, was at 50% of its storage capacity. The drought, combined with record-high temperatures, caused an estimated $1.3 billion in economic losses and threatened local water utilities’ ability to meet demand for four million people. Similar drought conditions unfolded in 2011-2012, during which numerous Water Trends Georgia counties were declared disaster zones. The Chattahoochee River, its tributaries, and Reduced rain affected recharge of the surface-water- Lake Lanier provide water to most of the dependent reservoir. It reduced flows, dried tributaries, “There is nothing simple, nothing one sub-basin Atlanta and Columbus metro populations. The and caused ecological damage in a landscape already river is the most heavily used water resource in affected by urbanization, impervious cover, and reduced can do to solve the problem. -

List of TMDL Implementation Plans with Tmdls Organized by Basin
Latest 305(b)/303(d) List of Streams List of Stream Reaches With TMDLs and TMDL Implementation Plans - Updated June 2011 Total Maximum Daily Loadings TMDL TMDL PLAN DELIST BASIN NAME HUC10 REACH NAME LOCATION VIOLATIONS TMDL YEAR TMDL PLAN YEAR YEAR Altamaha 0307010601 Bullard Creek ~0.25 mi u/s Altamaha Road to Altamaha River Bio(sediment) TMDL 2007 09/30/2009 Altamaha 0307010601 Cobb Creek Oconee Creek to Altamaha River DO TMDL 2001 TMDL PLAN 08/31/2003 Altamaha 0307010601 Cobb Creek Oconee Creek to Altamaha River FC 2012 Altamaha 0307010601 Milligan Creek Uvalda to Altamaha River DO TMDL 2001 TMDL PLAN 08/31/2003 2006 Altamaha 0307010601 Milligan Creek Uvalda to Altamaha River FC TMDL 2001 TMDL PLAN 08/31/2003 Altamaha 0307010601 Oconee Creek Headwaters to Cobb Creek DO TMDL 2001 TMDL PLAN 08/31/2003 Altamaha 0307010601 Oconee Creek Headwaters to Cobb Creek FC TMDL 2001 TMDL PLAN 08/31/2003 Altamaha 0307010602 Ten Mile Creek Little Ten Mile Creek to Altamaha River Bio F 2012 Altamaha 0307010602 Ten Mile Creek Little Ten Mile Creek to Altamaha River DO TMDL 2001 TMDL PLAN 08/31/2003 Altamaha 0307010603 Beards Creek Spring Branch to Altamaha River Bio F 2012 Altamaha 0307010603 Five Mile Creek Headwaters to Altamaha River Bio(sediment) TMDL 2007 09/30/2009 Altamaha 0307010603 Goose Creek U/S Rd. S1922(Walton Griffis Rd.) to Little Goose Creek FC TMDL 2001 TMDL PLAN 08/31/2003 Altamaha 0307010603 Mushmelon Creek Headwaters to Delbos Bay Bio F 2012 Altamaha 0307010604 Altamaha River Confluence of Oconee and Ocmulgee Rivers to ITT Rayonier -

Rule 391-3-6-.03. Water Use Classifications and Water Quality Standards
Presented below are water quality standards that are in effect for Clean Water Act purposes. EPA is posting these standards as a convenience to users and has made a reasonable effort to assure their accuracy. Additionally, EPA has made a reasonable effort to identify parts of the standards that are not approved, disapproved, or are otherwise not in effect for Clean Water Act purposes. Rule 391-3-6-.03. Water Use Classifications and Water Quality Standards ( 1) Purpose. The establishment of water quality standards. (2) W ate r Quality Enhancement: (a) The purposes and intent of the State in establishing Water Quality Standards are to provide enhancement of water quality and prevention of pollution; to protect the public health or welfare in accordance with the public interest for drinking water supplies, conservation of fish, wildlife and other beneficial aquatic life, and agricultural, industrial, recreational, and other reasonable and necessary uses and to maintain and improve the biological integrity of the waters of the State. ( b) The following paragraphs describe the three tiers of the State's waters. (i) Tier 1 - Existing instream water uses and the level of water quality necessary to protect the existing uses shall be maintained and protected. (ii) Tier 2 - Where the quality of the waters exceed levels necessary to support propagation of fish, shellfish, and wildlife and recreation in and on the water, that quality shall be maintained and protected unless the division finds, after full satisfaction of the intergovernmental coordination and public participation provisions of the division's continuing planning process, that allowing lower water quality is necessary to accommodate important economic or social development in the area in which the waters are located. -

River Clean-Up Guru, Bobby Marie…
River Clean-Up Guru, Bobby Marie… 1/11/2012 - Chattahoochee River 1/14/2012 – Etowah River 1/14/2012 – Coosa River 1/14/2012 – Oostanaula River 2/8/2012 – Peachtree Creek, South Fork 2/15/2012 – Peachtree Creek, North Fork 2/29/2012 – Suwannee River 4/21/2012 – Little River 5/16/2012 – Nickajack Creek 6/17/2012 – Altamaha River 8/8/2012 – Amicalola Creek 9/8/2012 – South River To view more 12 in 2012 finishers, go here. 1/11/2012 – Chattahoochee River Good Morning, I and two others paddled upstream on the Chattahoochee from Jones Bridge for about 4 miles then back down on a cold January afternoon on the 11th. It rained on us a couple of times, but the paddling kept us warm. We passed empty golf courses and leafless trees. We did see several herons and a couple of raptors hunting the river. Bobby Marie 1/14/2012 – Etowah, Coosa, Oostanala Rivers On January 14th, I joined Joe Cook and about 100 others on the CRBI Polar Bear Paddle over by Rome, GA. In one day I paddled 3 rivers, the Etowah for the major portion of the trip, then took two side paddles, upstream on the Oostanala for 30 minutes and then down and back up the Coosa for 30 minutes. When you reach the confluence of these three rivers you can look down and see the difference in the waters. The Etowah was greenish and the Oostanala was very brown and the Coosa was a mixture of the two! I only saw one BIG cooter on the bank in the sun the whole day. -

0429Lanierdoc
Planned Primary Project Name Corps District Work Description Allocation State ($000) Work performed with recovery funds includes the control and removal of nuisance vegetation from the upper St. Johns River which serves as a nursery area for vegetation which floats downstream into the St. Johns River Federal Navigation Project. This work will keep the project channel open for navigation and to ensure public safety. This vegetation also displaces native species, changes ecosystem structure and alters ecological functions potentially impacting threatened and endangered species. Work will be FL REMOVAL OF AQUATIC GROWTH, FL JACKSONVILLE performed by hired contract. 225 Award a contract for replacement of critical equipment used to conduct invasive vegetation operations in the Jacksonville District. These operations include survey and monitoring of vegetation in the St. Johns River and Lake Okeechobee. The operations keep the principal navigable waterways and structures open for navigation and to ensure public safety. Additionally, this vegetation displaces native species, changes ecosystem structure and alters ecological functions potentially impacting threatened and endangered FL REMOVAL OF AQUATIC GROWTH, FL JACKSONVILLE species 225 Snagging, clearing, and removal of fallen trees, stumps and other debris from the Withlachoochee River FL WITHLACOOCHEE RIVER, FL JACKSONVILLE Federal navigation Project for the purpose of ensuring navigation and public safety. 250 Update inundation mapping below project for dam safety, flood damage reduction and emergency action GA ALLATOONA LAKE, GA MOBILE plans in order to improve emergency response to flood events and reduce risk to public. 350 Hire additional contract employees to provide increased maintenance support for project facilities.These activities will provide the public a safe and enjoyable recreational experience at the project. -

Trophic State and Metabolism in a Southeastern Piedmont Reservoir
TROPHIC STATE AND METABOLISM IN A SOUTHEASTERN PIEDMONT RESERVOIR by Mary Callie Mayhew (Under the direction of Todd C. Rasmussen) Abstract Lake Sidney Lanier is a valuable water resource in the rapidly developing region north of Atlanta, Georgia, USA. The reservoir has been managed by the U.S Army Corps of Engineers for multiple purposes since its completion in 1958. Since approximately 1990, Lake Lanier has been central to series of lawsuits in the “Eastern Water Wars” between Georgia, Alabama and Florida due to its importance as a water-storage facility within the Apalachicola-Chattahoochee-Flint River Basin. Of specific importance is the need to protect lake water quality to satisfy regional water supply demands, as well as for recreational and environmental purposes. Recently, chlorophyll a levels have exceeded state water-quality standards. These excee- dences have prompted the Georgia Environmental Protection Division to develop Total Max- imum Daily Loads for phosphorus in Lake Lanier. While eutrophication in Southeastern Piedmont impoundments is a regional problem, nutrient cycling in these lakes does not appear to behave in a manner consistent with lakes in higher latitudes, and, hence, may not respond to nutrient-abatement strategies developed elsewhere. Although phosphorus loading to Southeastern Piedmont waterbodies is high, soluble reac- tive phosphorus concentrations are generally low and phosphorus exports from the reservoir are only a small fraction of input loads. The prevailing hypothesis is that ferric oxides in the iron-rich, clay soils of the Southeastern Piedmont effectively sequester phosphorus, which then settle into the lake benthos. Yet, seasonal algal blooms suggest the presence of internal cycling driven by uncertain mechanisms. -

Update of the Water Control Manual
91154 Federal Register / Vol. 81, No. 242 / Friday, December 16, 2016 / Notices ADDRESSES section of this notice. Before Jim Woodruff Lock and Dam and Lake total of 705 mgd to a range of 597–621 including your address, phone number, Seminole. mgd—242 mgd from Lake Lanier email address, or any other personal The purpose and need for the federal (instead of 297 mgd) and 355–379 mgd identifying information in your action is to determine how federal downstream (instead of 408 mgd)— comment, you should be aware that projects in the ACF Basin should be through the year 2050 rather than 2040 your entire comment—including your operated for their authorized purposes, as specified in the 2013 request. personal identifying information—may in light of current conditions and USACE’s objectives for the Master be made available to the public at any applicable law, and to implement those Manual are to develop a water control time. While you can request us to operations through updated water plan that meets the existing water withhold your personal identifying control plans and manuals. The resource needs of the basin, fulfills its information from public review, we proposed action will result in an responsibilities in operating for the cannot guarantee that we will be able to updated Master Manual and individual authorized project purposes, and do so. project water control manuals (WCMs) complies with all pertinent laws. The Dated: December 8, 2016. that comply with existing USACE FEIS presents the results of USACE’s regulations and reflect operations under analysis of the environmental effects of Mark Harberg, existing congressional authorizations, the Proposed Action Alternative (PAA) Missouri River Recovery Program Manager, taking into account changes in basin that the USACE believes accomplishes U.S. -
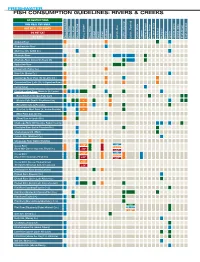
Fish Consumption Guidelines: Rivers & Creeks
FRESHWATER FISH CONSUMPTION GUIDELINES: RIVERS & CREEKS NO RESTRICTIONS ONE MEAL PER WEEK ONE MEAL PER MONTH DO NOT EAT NO DATA Bass, LargemouthBass, Other Bass, Shoal Bass, Spotted Bass, Striped Bass, White Bass, Bluegill Bowfin Buffalo Bullhead Carp Catfish, Blue Catfish, Channel Catfish,Flathead Catfish, White Crappie StripedMullet, Perch, Yellow Chain Pickerel, Redbreast Redhorse Redear Sucker Green Sunfish, Sunfish, Other Brown Trout, Rainbow Trout, Alapaha River Alapahoochee River Allatoona Crk. (Cobb Co.) Altamaha River Altamaha River (below US Route 25) Apalachee River Beaver Crk. (Taylor Co.) Brier Crk. (Burke Co.) Canoochee River (Hwy 192 to Lotts Crk.) Canoochee River (Lotts Crk. to Ogeechee River) Casey Canal Chattahoochee River (Helen to Lk. Lanier) (Buford Dam to Morgan Falls Dam) (Morgan Falls Dam to Peachtree Crk.) * (Peachtree Crk. to Pea Crk.) * (Pea Crk. to West Point Lk., below Franklin) * (West Point dam to I-85) (Oliver Dam to Upatoi Crk.) Chattooga River (NE Georgia, Rabun County) Chestatee River (below Tesnatee Riv.) Chickamauga Crk. (West) Cohulla Crk. (Whitfield Co.) Conasauga River (below Stateline) <18" Coosa River <20" 18 –32" (River Mile Zero to Hwy 100, Floyd Co.) ≥20" >32" <18" Coosa River <20" 18 –32" (Hwy 100 to Stateline, Floyd Co.) ≥20" >32" Coosa River (Coosa, Etowah below <20" Thompson-Weinman dam, Oostanaula) ≥20" Coosawattee River (below Carters) Etowah River (Dawson Co.) Etowah River (above Lake Allatoona) Etowah River (below Lake Allatoona dam) Flint River (Spalding/Fayette Cos.) Flint River (Meriwether/Upson/Pike Cos.) Flint River (Taylor Co.) Flint River (Macon/Dooly/Worth/Lee Cos.) <16" Flint River (Dougherty/Baker Mitchell Cos.) 16–30" >30" Gum Crk. -

Georgia Cobb Marietta
Form 10-306 UNITED STATES DEPARTMENT OF THE INTERIOR (Oct. 1972) NATIONAL PARK SERVICE Georgia NATIONAL REGISTER OF HISTORIC PLACES Cobb INVENTORY - NOMINATION FORM FOR NPS USE ONLY FOR FEDERAL PROPERTIES ENTRY DATE (Type all entries - complete applicable sections) COMMON: _JVr ~~ " ~ • : - Kennesaw Mountain National:Battlefield Park AND/OR HISTORIC: STREET AND NUMBER: P. O. : BOK 1167 On western edge of Marietta, GA^just .of£ US CITY OR TOWN: CONGRESSIONAL DISTRICT: Marietta 7th STATE: COUNTY: , CODE Georgia : 137: . Cobb 067 ACCESSIBLE OWNERSHIP STATUS TO THE PUBLIC District | | Building L7Q Public Public Acquisition: Occupied Yes: Site' Q Structure L~3 Private Q In Process Unoccupied Qtl Restricted Q Object C] Both Q Being Considered Preservation work | | Unrestricted- in progress PRESENT USE (Check One or More as Appropriate) Q Agricultural [_J Government | | Transportation Comments t - [~~1 Commercial | | Industrial Q Private Residence Other fSpec/fy; [ ] Educational [^Military Q Religious | 1 Entertainment [~~| Museum I | Scientific Ui IU National Park ^Service, Kenne'saw Mountain National. Battlefield Park REGIONAL HEADQUARTERS: (I! applicable) STREET AMD NUMBER: Southeast Region Cl TY OR TOWN: Atlanta COURTHOUSE, REGISTRY OF DEEDS. ETC: Cobb County Courthouse STREET AND NUMBER: 177 Washington Avenue, N. ; E CITY_OR TOWN: Marietta TITLE OF SURVEY: None DATE OF SURVEY: DEPOSITORY FOR SURVEY RECORDS: STREET AND NUMBER: CITY OR TOWN: (Check One) f~l Excellent Stl Good Q Fair I | Deteriorated [ | Ruin* Q Unexpoted CONDITION (Check One) (Check One) (2Q Altered Q Unaltered Q Moved (X) Original Site DESCRIBE THE PRESENT AND ORIGINAL (it known) PHYSICAL APPEARANCE Kennesaw Mountain is an erosional granitic hornblende remnant located in the upper Georgia Piedmont. -

Fecal Coliform TMDL Report
Total Maximum Daily Load Evaluation for Nineteen Stream Segments in the Tennessee River Basin for Fecal Coliform Submitted to: The U.S. Environmental Protection Agency Region 4 Atlanta, Georgia Submitted by: The Georgia Department of Natural Resources Environmental Protection Division Atlanta, Georgia January 2004 Total Maximum Daily Load Evaluation January 2004 Tennessee River Basin (Fecal coliform) Table of Contents Section Page EXECUTIVE SUMMARY ............................................................................................................. iv 1.0 INTRODUCTION ................................................................................................................... 1 1.1 Background ....................................................................................................................... 1 1.2 Watershed Description......................................................................................................1 1.3 Water Quality Standard.....................................................................................................5 2.0 WATER QUALITY ASSESSMENT ........................................................................................ 8 3.0 SOURCE ASSESSMENT ...................................................................................................... 9 3.1 Point Source Assessment ................................................................................................. 9 3.2 Nonpoint Source Assessment........................................................................................ -

2018 Integrated 305(B)
2018 Integrated 305(b)/303(d) List - Streams Reach Name/ID Reach Location/County River Basin/ Assessment/ Cause/ Size/Unit Category/ Notes Use Data Provider Source Priority Alex Creek Mason Cowpen Branch to Altamaha Not Supporting DO 3 4a TMDL completed DO 2002. Altamaha River GAR030701060503 Wayne Fishing 1,55,10 NP Miles Altamaha River Confluence of Oconee and Altamaha Supporting 72 1 TMDL completed TWR 2002. Ocmulgee Rivers to ITT Rayonier GAR030701060401 Appling, Wayne, Jeff Davis Fishing 1,55 Miles Altamaha River ITT Rayonier to Penholoway Altamaha Assessment 20 3 TMDL completed TWR 2002. More data need to Creek Pending be collected and evaluated before it can be determined whether the designated use of Fishing is being met. GAR030701060402 Wayne Fishing 10,55 Miles Altamaha River Penholoway Creek to Butler Altamaha Supporting 27 1 River GAR030701060501 Wayne, Glynn, McIntosh Fishing 1,55 Miles Beards Creek Chapel Creek to Spring Branch Altamaha Not Supporting Bio F 7 4a TMDL completed Bio F 2017. GAR030701060308 Tattnall, Long Fishing 4 NP Miles Beards Creek Spring Branch to Altamaha Altamaha Not Supporting Bio F 11 4a TMDL completed Bio F in 2012. River GAR030701060301 Tattnall Fishing 1,55,10,4 NP, UR Miles Big Cedar Creek Griffith Branch to Little Cedar Altamaha Assessment 5 3 This site has a narrative rank of fair for Creek Pending macroinvertebrates. Waters with a narrative rank of fair will remain in Category 3 until EPD completes the reevaluation of the metrics used to assess macroinvertebrate data. GAR030701070108 Washington Fishing 59 Miles Big Cedar Creek Little Cedar Creek to Ohoopee Altamaha Not Supporting DO, FC 3 4a TMDLs completed DO 2002 & FC (2002 & 2007).