Organophosphate and N-Methyl Carbamate 03-12-2010.Xlsx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
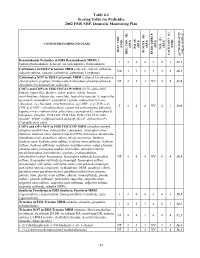
2002 NRP Section 6, Tables 6.1 Through
Table 6.1 Scoring Table for Pesticides 2002 FSIS NRP, Domestic Monitoring Plan } +1 0.05] COMPOUND/COMPOUND CLASS * ) (EPA) (EPA) (EPA) (EPA) (EPA) (FSIS) (FSIS) PSI (P) TOX.(T) L-1 HIST. VIOL. BIOCON. (B) {[( (2*R+P+B)/4]*T} REG. CON. (R) * ENDO. DISRUP. LACK INFO. (L) LACK INFO. {[ Benzimidazole Pesticides in FSIS Benzimidazole MRM (5- 131434312.1 hydroxythiabendazole, benomyl (as carbendazim), thiabendazole) Carbamates in FSIS Carbamate MRM (aldicarb, aldicarb sulfoxide, NA44234416.1 aldicarb sulfone, carbaryl, carbofuran, carbofuran 3-hydroxy) Carbamates NOT in FSIS Carbamate MRM (carbaryl 5,6-dihydroxy, chlorpropham, propham, thiobencarb, 4-chlorobenzylmethylsulfone,4- NT 4 1 3 NV 4 4 13.8 chlorobenzylmethylsulfone sulfoxide) CHC's and COP's in FSIS CHC/COP MRM (HCB, alpha-BHC, lindane, heptachlor, dieldrin, aldrin, endrin, ronnel, linuron, oxychlordane, chlorpyrifos, nonachlor, heptachlor epoxide A, heptachlor epoxide B, endosulfan I, endosulfan I sulfate, endosulfan II, trans- chlordane, cis-chlordane, chlorfenvinphos, p,p'-DDE, p, p'-TDE, o,p'- 3444NV4116.0 DDT, p,p'-DDT, carbophenothion, captan, tetrachlorvinphos [stirofos], kepone, mirex, methoxychlor, phosalone, coumaphos-O, coumaphos-S, toxaphene, famphur, PCB 1242, PCB 1248, PCB 1254, PCB 1260, dicofol*, PBBs*, polybrominated diphenyl ethers*, deltamethrin*) (*identification only) COP's and OP's NOT in FSIS CHC/COP MRM (azinphos-methyl, azinphos-methyl oxon, chlorpyrifos, coumaphos, coumaphos oxon, diazinon, diazinon oxon, diazinon met G-27550, dichlorvos, dimethoate, dimethoate -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Lifetime Organophosphorous Insecticide Use Among Private Pesticide Applicators in the Agricultural Health Study
Journal of Exposure Science and Environmental Epidemiology (2012) 22, 584 -- 592 & 2012 Nature America, Inc. All rights reserved 1559-0631/12 www.nature.com/jes ORIGINAL ARTICLE Lifetime organophosphorous insecticide use among private pesticide applicators in the Agricultural Health Study Jane A. Hoppin1, Stuart Long2, David M. Umbach3, Jay H. Lubin4, Sarah E. Starks5, Fred Gerr5, Kent Thomas6, Cynthia J. Hines7, Scott Weichenthal8, Freya Kamel1, Stella Koutros9, Michael Alavanja9, Laura E. Beane Freeman9 and Dale P. Sandler1 Organophosphorous insecticides (OPs) are the most commonly used insecticides in US agriculture, but little information is available regarding specific OP use by individual farmers. We describe OP use for licensed private pesticide applicators from Iowa and North Carolina in the Agricultural Health Study (AHS) using lifetime pesticide use data from 701 randomly selected male participants collected at three time periods. Of 27 OPs studied, 20 were used by 41%. Overall, 95% had ever applied at least one OP. The median number of different OPs used was 4 (maximum ¼ 13). Malathion was the most commonly used OP (74%) followed by chlorpyrifos (54%). OP use declined over time. At the first interview (1993--1997), 68% of participants had applied OPs in the past year; by the last interview (2005--2007), only 42% had. Similarly, median annual application days of OPs declined from 13.5 to 6 days. Although OP use was common, the specific OPs used varied by state, time period, and individual. Much of the variability in OP use was associated with the choice of OP, rather than the frequency or duration of application. -

Quantum Chemical Study of the Thermochemical Properties of Organophosphorous Compounds A
QUANTUM CHEMICAL STUDY OF THE THERMOCHEMICAL PROPERTIES OF ORGANOPHOSPHOROUS COMPOUNDS A. Khalfa, M. Ferrari, R. Fournet, B. Sirjean, L. Verdier, Pierre-Alexandre Glaude To cite this version: A. Khalfa, M. Ferrari, R. Fournet, B. Sirjean, L. Verdier, et al.. QUANTUM CHEMICAL STUDY OF THE THERMOCHEMICAL PROPERTIES OF ORGANOPHOSPHOROUS COMPOUNDS. Journal of Physical Chemistry A, American Chemical Society, 2015, 119 (42), pp.10527-10539. 10.1021/acs.jpca.5b07071. hal-01241498 HAL Id: hal-01241498 https://hal.archives-ouvertes.fr/hal-01241498 Submitted on 10 Dec 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. QUANTUM CHEMICAL STUDY OF THE THERMOCHEMICAL PROPERTIES OF ORGANOPHOSPHOROUS COMPOUNDS A. Khalfa, M. Ferrari1, R. Fournet1, B. Sirjean1, L. Verdier2, P.A. Glaude1 1Laboratoire Réactions et Génie des Procédés, Université de Lorraine, CNRS, 1 rue Grandville, BP 20451, 54001 NANCY Cedex, France, 2DGA Maîtrise NRBC, Site du Bouchet, 5 rue Lavoisier, BP n°3, 91710 Vert le Petit, France Abstract Organophosphorous compounds are involved in many toxic compounds such as fungicides, pesticides, or chemical warfare nerve agents. The understanding of the decomposition chemistry of these compounds in the environment is largely limited by the scarcity of thermochemical data. -

Fate and Effects of Azinphos-Methyl in a Flow-Through Wetland in South
Environ. Sci. Technol. 2003, 37, 2139-2144 preventing it from entering downstream aquatic habitats (1, Fate and Effects of Azinphos-Methyl 2). The implementation of retention ponds in agricultural in a Flow-Through Wetland in South watersheds was mentioned by Scott et al. (3) as one strategy to reduce the amount and toxicity of runoff-related insecticide Africa pollution discharging into estuaries. The usefulness of aquatic plants for removal of insecticides from water has been shown ,² ² in an indoor microcosm study (4), and the effects of the RALF SCHULZ,* CHRISTINA HAHN, organophosphate phorate have been assessed using littoral ERIN R. BENNETT,² mesocosms in South Dakota wetlands (5). However, infor- JAMES M. DABROWSKI,² mation about the fate or effects of spray drift-borne GERALDINE THIERE,² AND SUE K. C. PEALL³ insecticide input in constructed wetlands is limited. Processes important for removal of nonpoint-source Department of Zoology, Private Bag X1, University of pesticide pollution in wetlands may include adsorption, Stellenbosch, Matieland 7602, South Africa, and Forensic Chemistry Laboratory, Department of Health, decomposition, hydrolysis, microbial metabolism, photolysis, Cape Town 8000, South Africa and volatilization (6). The macrophytes present in the wetland may play an important role in providing an increased surface area for sorption as well as for microbial activity (7). Further- more, they may contribute directly to metabolism (8). Our knowledge about the effectiveness of constructed Spray drift is an important route for nonpoint-source wetlands in retaining agricultural nonpoint-source pesticide pesticide pollution of aquatic habitats (9, 10). Specifically, pollution is limited. A 0.44-ha vegetated wetland built orchard applications result in a large amount of drift due to small droplet size and the trajectory of release (11). -

Code Chemical P026 1-(O-Chlorophenyl)Thiourea P081 1
Code Chemical P026 1-(o-Chlorophenyl)thiourea P081 1,2,3-Propanetriol, trinitrate (R) P042 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, (R)- P067 1,2-Propylenimine P185 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)- carbonyl]oxime 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a,-hexahydro-, P004 (1alpha,4alpha, 4abeta,5alpha,8alpha,8abeta)- 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a-hexahydro-, P060 (1alpha,4alpha, 4abeta,5beta,8beta,8abeta)- P002 1-Acetyl-2-thiourea P048 2,4-Dinitrophenol P051 2,7:3,6-Dimethanonaphth [2,3-b]oxirene, 3,4,5,6,9,9 -hexachloro-1a,2,2a,3,6,6a,7,7a- octahydro-, (1aalpha,2beta,2abeta,3alpha,6alpha,6abeta,7 beta, 7aalpha)-, & metabolites 2,7:3,6-Dimethanonaphth[2,3-b]oxirene, 3,4,5,6,9,9- hexachloro-1a,2,2a,3,6,6a,7,7a- P037 octahydro-, (1aalpha,2beta,2aalpha,3beta,6beta,6aalpha,7 beta, 7aalpha)- P045 2-Butanone, 3,3-dimethyl-1-(methylthio)-, O-[methylamino)carbonyl] oxime P034 2-Cyclohexyl-4,6-dinitrophenol 2H-1-Benzopyran-2-one, 4-hydroxy-3-(3-oxo-1- phenylbutyl)-, & salts, when present at P001 concentrations greater than 0.3% P069 2-Methyllactonitrile P017 2-Propanone, 1-bromo- P005 2-Propen-1-ol P003 2-Propenal P102 2-Propyn-1-ol P007 3(2H)-Isoxazolone, 5-(aminomethyl)- P027 3-Chloropropionitrile P047 4,6-Dinitro-o-cresol, & salts P059 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro- 3a,4,7,7a-tetrahydro- P008 4-Aminopyridine P008 4-Pyridinamine P007 5-(Aminomethyl)-3-isoxazolol 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- -

Parathion-Methyl
FAO SPECIFICATIONS AND EVALUATIONS FOR PLANT PROTECTION PRODUCTS PARATHION-METHYL O,O-dimethyl O-4-nitrophenyl phosphorothioate 2001 TABLE OF CONTENTS PARATHION-METHYL Page DISCLAIMER 3 INTRODUCTION 4 PART ONE 5 SPECIFICATIONS FOR PARATHION-METHYL PARATHION-METHYL INFORMATIONERROR! BOOKMARK NOT DEFINED. PARATHION-METHYL TECHNICAL MATERIAL 6 PARATHION-METHYL TECHNICAL CONCENTRATE 8 PARATHION-METHYL EMULSIFIABLE CONCENTRATE 10 PART TWO 13 2001 EVALUATION REPORT ON PARATHION-METHYL 14 Page 2 of 31 PARATHION-METHYL SPECIFICATIONS 2001 Disclaimer1 FAO specifications are developed with the basic objective of ensuring that pesticides complying with them are satisfactory for the purpose for which they are intended so that they may serve as an international point of reference. The specifications do not constitute an endorsement or warranty of the use of a particular pesticide for a particular purpose. Neither do they constitute a warranty that pesticides complying with these specifications are suitable for the control of any given pest, or for use in a particular area. Owing to the complexity of the problems involved, the suitability of pesticides for a particular application must be decided at the national or provincial level. Furthermore, the preparation and use of pesticides complying with these specifications are not exempted from any safety regulation or other legal or administrative provision applicable thereto. FAO shall not be liable for any injury, loss, damage or prejudice of any kind that may be suffered as a result of the preparation, transportation, sale or use of pesticides complying with these specifications. Additionally, FAO wishes to alert users of specifications to the fact that improper field mixing and/or application of pesticides can result in either a lowering or complete loss of efficacy. -

Pesticides and Toxic Substances
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON D.C., 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES MEMORANDUM DATE: July 31, 2006 SUBJECT: Finalization of Interim Reregistration Eligibility Decisions (IREDs) and Interim Tolerance Reassessment and Risk Management Decisions (TREDs) for the Organophosphate Pesticides, and Completion of the Tolerance Reassessment and Reregistration Eligibility Process for the Organophosphate Pesticides FROM: Debra Edwards, Director Special Review and Reregistration Division Office of Pesticide Programs TO: Jim Jones, Director Office of Pesticide Programs As you know, EPA has completed its assessment of the cumulative risks from the organophosphate (OP) class of pesticides as required by the Food Quality Protection Act of 1996. In addition, the individual OPs have also been subject to review through the individual- chemical review process. The Agency’s review of individual OPs has resulted in the issuance of Interim Reregistration Eligibility Decisions (IREDs) for 22 OPs, interim Tolerance Reassessment and Risk Management Decisions (TREDs) for 8 OPs, and a Reregistration Eligibility Decision (RED) for one OP, malathion.1 These 31 OPs are listed in Appendix A. EPA has concluded, after completing its assessment of the cumulative risks associated with exposures to all of the OPs, that: (1) the pesticides covered by the IREDs that were pending the results of the OP cumulative assessment (listed in Attachment A) are indeed eligible for reregistration; and 1 Malathion is included in the OP cumulative assessment. However, the Agency has issued a RED for malathion, rather than an IRED, because the decision was signed on the same day as the completion of the OP cumulative assessment. -

Combined Pre-And Posttreatment of Paraoxon Exposure
molecules Article Combined Pre- and Posttreatment of Paraoxon Exposure Dietrich E Lorke 1,2,* , Syed M Nurulain 3 , Mohamed Y Hasan 4, Kamil Kuˇca 5 and Georg A Petroianu 2,6 1 Department of Anatomy and Cellular Biology, College of Medicine and Health Sciences, Khalifa University, P O Box 127788, Abu Dhabi, UAE 2 Herbert Wertheim College of Medicine, Department of Cellular Biology & Pharmacology, Florida International University, University Park GL 495, 11200 SW 8th St, Miami, FL 33199, USA; [email protected] 3 Bio Science Department, COMSATS Institute of Information Technology, Bio Sciences Block, CUI, Park Road, Tarlai Kalan, Islamabad 45550, Pakistan; [email protected] 4 Department of Pharmacology & Therapeutics, College of Medicine and Health Sciences, UAE University, Al Ain 15551, UAE; [email protected] 5 Department of Chemistry, Faculty of Science, University of Hradec Kralove, Rokitanského 62/26, 500 03 Hradec Kralove, Czech Republic; [email protected] 6 Department of Pharmacology, College of Medicine and Health Sciences, Khalifa University, P O Box 127788, Abu Dhabi, UAE * Correspondence: [email protected]; Tel.: +971-2-501-8381 Academic Editors: Pascal Houzé and Frédéric J. Baud Received: 5 March 2020; Accepted: 25 March 2020; Published: 27 March 2020 Abstract: Aims: Organophosphates (OPCs), useful agents as pesticides, also represent a serious health hazard. Standard therapy with atropine and established oxime-type enzyme reactivators is unsatisfactory. Experimental data indicate that superior therapeutic results can be obtained when reversible cholinesterase inhibitors are administered before OPC exposure. Comparing the protective efficacy of five such cholinesterase inhibitors (physostigmine, pyridostigmine, ranitidine, tacrine, or K-27), we observed best protection for the experimental oxime K-27. -

Phorate Interim AEGL Document
1 2 3 4 ACUTE EXPOSURE GUIDELINE LEVELS (AEGLs) 5 FOR 6 PHORATE 7 (CAS Reg. No. 298-02-2) 8 9 INTERIM 10 11 12 13 14 15 16 17 18 PHORATE Interim 09-2009; Page 2 of 30 1 2 ACUTE EXPOSURE GUIDELINE LEVELS (AEGLs) 3 FOR 4 PHORATE 5 (CAS Reg. No. 298-02-2) 6 7 8 9 10 INTERIM 11 12 13 14 15 16 17 18 19 20 21 22 23 PHORATE Interim 09-2009; Page 3 of 30 1 2 3 PREFACE 4 5 Under the authority of the Federal Advisory Committee Act (FACA) P. L. 92-463 of 6 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous 7 Substances (NAC/AEGL Committee) has been established to identify, review and interpret 8 relevant toxicologic and other scientific data and develop AEGLs for high priority, acutely toxic 9 chemicals. 10 11 AEGLs represent threshold exposure limits for the general public and are applicable to 12 emergency exposure periods ranging from 10 minutes to 8 hours. Three levels C AEGL-1, 13 AEGL-2 and AEGL-3 C are developed for each of five exposure periods (10 and 30 minutes, 1 14 hour, 4 hours, and 8 hours) and are distinguished by varying degrees of severity of toxic effects. 15 The three AEGLs are defined as follows: 16 17 AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per 18 cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general 19 population, including susceptible individuals, could experience notable discomfort, irritation, or 20 certain asymptomatic, non-sensory effects. -

Biosensors for Detection of Organophosphate Exposure by New Diethyl Thiophosphate-Specifc Aptamer
Biosensors for Detection of Organophosphate Exposure by New Diethyl Thiophosphate-Specic Aptamer Napachanok Mongkoldhumrongkul Swainson Kasetsart University - Bangkhen Campus: Kasetsart University Chonnikarn Saikaew Kasetsart University - Bangkhen Campus: Kasetsart University Kanyanat Theeraraksakul Kasetsart University - Bangkhen Campus: Kasetsart University Pongsakorn Aiemderm Kasetsart University - Bangkhen Campus: Kasetsart University Rimdusit Pakjira Department of Disease Control Charoenkwan Kraiya Chula: Chulalongkorn University Sasimanas Unajak Kasetsart University - Bangkhen Campus: Kasetsart University kiattawee choowongkomon ( [email protected] ) Kasetsart University https://orcid.org/0000-0002-2421-7859 Research Article Keywords: Aptasensor, organophosphate metabolites, diethyl thiophosphate, electrochemical impedance spectroscopy, capillary electrophoresis Posted Date: February 23rd, 2021 DOI: https://doi.org/10.21203/rs.3.rs-217995/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at Biotechnology Letters on July 6th, 2021. See the published version at https://doi.org/10.1007/s10529-021-03158-2. Page 1/17 Abstract Objective An aptamer specically binding to diethyl thiophosphate (DETP) was constructed and incorporated in an optical sensor and electrochemical impedance spectroscopy (EIS) to enable the specic measurement of DETP as a metabolite and a biomarker of exposure to organophosphates. Results DETP-bound aptamer was selected from the library using capillary electrophoresis-systematic evolution of ligands by exponential enrichment (CE-SELEX). A colorimetric method revealed the aptamer had the highest anity to DETP with a mean Kd value (± SD) of 0.103 ± 0.014 µM. Changes in resistance using EIS showed selectivity of the aptamer for DETP higher than for dithiophosphate (DEDTP) and diethyl phosphate (DEP) which have similar structure and are metabolites of some of the same organophosphates. -

Visualisation of DCP, a Nerve Agent Mimic, in Catfish Brain by a Simple
www.nature.com/scientificreports OPEN Visualisation of DCP, a nerve agent mimic, in Catfsh brain by a simple chemosensor Received: 25 October 2017 Himadri Sekhar Sarkar 1, Ayndrila Ghosh1, Sujoy Das1, Pulak Kumar Maiti2, Sudipta Maitra3, Accepted: 9 February 2018 Sukhendu Mandal 2 & Prithidipa Sahoo 1 Published: xx xx xxxx A chemosensor, 3-aminophenol-based rhodamine conjugate (ARC) has been developed for visualisation of diethylchlorophosphate (DCP), mimic of a chemical warfare agent, in Catfsh brain. The simple detection of DCP by “turn-on” fuorescence property of the chemosensor makes it unique for easy and rapid in vivo and in vitro detection of DCP with the detection limit of 5.6 nM. 1995, Te terrorist attack on Tokyo subway introduces the whole world with a new threat to mankind- Chemical Warfare Agents (CWAs)1–3. Te simple organophosphates, present in pesticides eventually become more pop- ular chemical weapon due to its very simple method of manufacturing, availability, and low cost along with its dispensability. Despite of its common use as a pesticide, various analogues of such organophosphates are found to be very potent nerve agent, which irreversibly damage functions of nerve cells. Among all the highly toxic, volatile nerve agents, Sarin (GB), Soman (GD) and Tabun (GA) are of most common. If the nerve agents are being inhaled or absorbed through skin, their reactive phosphate group irreversibly react with the hydroxyl group of cellular acetylcholinesterase, which is responsible for breaking down the acetylcholine neurotransmitter, leads to its inactivation. Te fatal consequences are neurological imbalance at cholinergic synapses, failure of several organs, paralysis of central nervous system and rapid death4,5.