Perrigo Annual Report 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Understanding the FTC's Monetary Equitable Remedies Under Section
Understanding the FTC’s monetary equitable remedies under Section 13(b) for antitrust violations By Gerald A. Stein, American Bar Association — Fall 2019 Until recently the Federal Trade Commission’s ability to seek monetary equitable remedies (particularly disgorgement and restitution) for alleged antitrust violations— whether ongoing, impending, or stale— went virtually unchallenged. After it first gained statutory authority under Section 13(b)1 of the FTC Act to bring suit in federal district court to preliminarily enjoin conduct that “is violating, or is about to violate” the antitrust laws, the FTC successfully convinced many federal courts that Section 13(b) also impliedly allowed the FTC to seek standalone permanent injunctions and monetary equitable remedies regardless of whether a defendant’s alleged anticompetitive conduct was ongoing or completed long ago.2 The FTC brought few antitrust cases in the years following the enactment for alleged antitrust violations pursuant to Section 13(b) where the alleged of Section 13(b) in 1973, and the agency formalized its conservative anticompetitive conduct was neither ongoing nor imminent. Next, in approach in its unanimously approved 2003 Policy Statement on Monetary FTC v. Credit Bureau Center LLC,5 the Seventh Circuit vacated an award for Equitable Remedies in Competition Cases. In 2012, however, the FTC restitution in a consumer protection case based on its determination that abruptly, and without providing an opportunity for public comment, Section 13(b) authorizes only restraining orders and injunctions. And, in a withdrew its 2003 Policy Statement, asserting that it created “an overly third case that is still pending, FTC v. -

Responding to Mylan's Inadequate Tender Offer
Responding To Mylan’s Inadequate Tender Offer: Perrigo’s Board Recommends That You Reject the Offer and Do Not Tender September 2015 Important Information Forward Looking Statements Certain statements in this presentation are forward-looking statements. These statements relate to future events or the Company’s future financial performance and involve known and unknown risks, uncertainties and other factors that may cause the actual results, levels of activity, performance or achievements of the Company or its industry to be materially different from those expressed or implied by any forward-looking statements. In some cases, forward-looking statements can be identified by terminology such as “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “potential” or other comparable terminology. The Company has based these forward-looking statements on its current expectations, assumptions, estimates and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond the Company’s control, including future actions that may be taken by Mylan in furtherance of its unsolicited offer. These and other important factors, including those discussed under “Risk Factors” in the Perrigo Company’s Form 10-K for the year ended June 27, 2015, as well as the Company’s subsequent filings with the Securities and Exchange -

2020 Annual Report Products
Products 2020 Annual Report From the CEO Dear Fellow Shareholders, Perrigo’s transformation to a pure-play Consumer Self-Care Company has come a long way LQMXVWWZRVKRUW\HDUV:HKDYHUHVWRUHGVXVWDLQDEOHWRSOLQHJURZWKGHOLYHUHGRQRXU¿QDQFLDO SURPLVHVUHFRQ¿JXUHGRXUSRUWIROLRRIEXVLQHVVHVXSGDWHGWKH,7LQIUDVWUXFWXUHDQGSURFHVVHV of the Company, expanded capacity, upgraded leadership talent, installed business intelligence capabilities, built a new product pipeline of over $500 million and re-instilled a sense of pride and energy among our 11,000 team members. Making this even more remarkable, is that we kept the WUDQVIRUPDWLRQRQWUDFNLQWKHIDFHRIWKHJOREDO&29,'SDQGHPLF,KRSH\RXDUHDVSURXGRI3HUULJR¶VJOREDOWHDP DV,DPIRUKRZWKH\ZRUNHGWRNHHSHDFKRWKHUVDIHNHSWRXUHVVHQWLDOSURGXFWVÀRZLQJDQGNHSWRXUWUDQVIRUPDWLRQ to a consumer self-care company on track through all of the personal and professional uncertainty that came their way LQ7KH\DUHKHURHV As a result of their efforts, Perrigo delivered strong net sales growth for the second year in a row in 2020 and World-wide Consumer sales reached a new record high. Equally important, the team stabilized adjusted operating income after a few years of decline even as we invested over $50 million in our business and overcame $35 million RIXQSODQQHGKHDGZLQGVGXHSULPDULO\WR&29,'UHODWHGVDIHW\FRVWVDQGEXVLQHVVLPSDFWIURPWKHZHDNFROG FRXJKDQGÀXVHDVRQUHODWHGWR&29,'¶VLPSDFWRQSXEOLFOLIH$OOLQDOOZHKDGDYHU\VWURQJ\HDU Our transformation efforts reached an essential milestone after the year closed when we announced the sale of RXU3UHVFULSWLRQ3KDUPDFHXWLFDOVEXVLQHVVWR$OWDULV&DSLWDO3DUWQHUV//&7KHWUDQVDFWLRQUHLQIRUFHVRXUDELOLW\ -

Pharma Pricing, Non-Profit Ties Get Increasing Scrutiny from Prosecutors
REGULATORY UPDATE REGULATORY UPDATE CONSUMER DRUGS Pediatric Rare Disease Voucher Japan Wants EMA To Stay In Digital Marketing: Health Care Brands’ Program Faces Expiration, p. 12 UK Post-Brexit, p. 15 Window Into Consumers’ Lives, p. 20 Pharma intelligence Pinkpink.pharmamedtechbi.comSheetVol. 78 / No. 38 September 19, 2016 informa Mylan NV was subpoenaed for material Pharma Pricing, Non-Profit Ties Get about the pricing of its generic doxycycline and communications with competitors. And Valeant Pharmaceuticals International Inc. is Increasing Scrutiny From Prosecutors facing several probes about its pricing and BRENDA SANDBURG [email protected] patient assistance programs (see chart, p. 5). Mylan’s doxycycline price increases were called out by Sen. Bernie Sanders, I-Vt., and Rep. Elijah Cummings, D-Md., in October 2014 when they sent letters to 14 generic drug makers about the pricing of their prod- ucts. They noted that from October 2013 to April 2014, the average price charged for a 500-count bottle of 100 mg tablets had risen from $20 to $1,829, an 8,281% increase. Mylan is now under fire for repeatedly rais- ing the price of its severe allergy treatment EpiPen (epinephrine), which has increased from about $100 for a two-pack in 2008 to more than $600. Members of Congress sent a flurry of letters to the company requesting an explanation for the price hikes. And on Sept. 6, New York Attorney Gen- Shutterstock: blvdone Shutterstock: eral Eric Schneiderman announced that his office has begun an investigation into rug makers have been unable to programs, contractual agreements with Mylan with regard to EpiPen, saying a pre- shake free of government inves- pharmacy benefit managers, support of liminary review revealed that Mylan may Dtigations of their marketing and non-profit organizations, and calculation have inserted potentially anticompetitive sales practices. -

Plaintiff Federal Trade Commission's Post-Trial Brief
Case 2:14-cv-05151-HB Document 403 Filed 03/23/18 Page 1 of 46 UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA FEDERAL TRADE COMMISSION, Plaintiff, Case Number: 2:14-CV-5151-HB v. ABBVIE INC., et al., Defendants. PLAINTIFF FEDERAL TRADE COMMISSION’S POST-TRIAL BRIEF Armine Black Daniel S. Bradley Rebecca L. Egeland Jordy J. Hur Garth W. Huston Hannah Lamb Heather M. Johnson Thomas D. Mays Patricia M. McDermott Markus H. Meier Kara L. Monahan Lauren Peay Peter J. Taylor Matthew B. Weprin James Weingarten Attorneys for Plaintiff FEDERAL TRADE COMMISSION 600 Pennsylvania Avenue, N.W. Washington, D.C. 20580 (202) 326-2569 [email protected] Case 2:14-cv-05151-HB Document 403 Filed 03/23/18 Page 2 of 46 TABLE OF CONTENTS TABLE OF AUTHORITIES ........................................................................................................ ii KEY PERSONNEL ...................................................................................................................... vi I. Defendants’ objectively baseless lawsuits used the governmental process as an anticompetitive weapon .................................................................................................... 2 A. Defendants enjoyed substantial benefits from the collateral injuries their baseless lawsuits inflicted on Teva and Perrigo ...................................................... 3 B. The evidence at trial supports liability even under Defendants’ erroneous view that PRE requires proof that Defendants knew their lawsuits were baseless ......... -

Perrigo Board of Directors
Perrigo Company plc Board of Directors and Their Compensation (as of August 7, 2019) Bradley A. Alford, 62, has been a director of Perrigo since February 2017. Mr. Alford joined Advent International Corporation, a global private equity firm, in 2014 as an Industry Advisor and moved to Operating Partner in March of 2016. From 2006 to 2013, Mr. Alford was Chairman and Chief Executive Officer of Nestlé USA. Mr. Alford also served as CEO and President of Nestlé Brands Company. He currently serves as a director of Avery Dennison Corporation since April 2010 and previously served as a director of Conagra Brands, Inc. from July 2015 to September 2018. Throughout his career, Mr. Alford has been focused on developing brands, initiatives to improve processes and facilitate best practices across an organization. Rolf A. Classon, 73, has been a director of Perrigo since May 2017. Mr. Classon served as Interim President and Chief Executive Officer of Hillenbrand Industries, a global diversified industrial company, from May 2005 until March 2006. From 2002 until June 2004, Mr. Classon served as Chairman of the Executive Committee of Bayer Healthcare AG, a subsidiary of Bayer AG. Mr. Classon served as President of Bayer Diagnostics from 1995 to 2002 and as Executive Vice President from 1991 to 1995. Prior to 1991, Mr. Classon held various management positions with Pharmacia Corporation. Mr. Classon serves as a director of Fresenius Medical Care AG and Co. since May 2012, and Catalent, Inc. since July 2014. Mr. Classon also served as a director of Hill-Rom Holdings, Inc., from July 2001 to March 2018, Aerocrine AB, Stockholm from May 2013 to July 2015 and Auxilium Pharmaceuticals from July 2005 to January 2015 and served as a director of Tecan Group, Ltd. -

View Annual Report
A Proven Formula for Delivering Superior Shareholder Value A A Proven Formula for Delivering Superior Shareholder Value Corporate Profile From its beginnings in 1887 as a packager of generic home remedies, Perrigo Company plc, headquartered in Ireland, has grown to become the world’s largest manufacturer of over-the-counter (OTC) products and supplier of infant formulas for the store brand market, and is now a top five global OTC consumer goods and pharmaceutical company. In addition to receiving royalties from Multiple Sclerosis drug Tysabri®, the Company is also a leading provider of branded OTC products and generic extended topical prescription products. Perrigo provides Quality Affordable Healthcare Products® across a wide variety of product categories and geographies, primarily in North America, Europe, and Australia, as well as in other markets, including Israel and China. Perrigo 1 Fellow Shareholders, On behalf of the Perrigo management team and the over 13,000 Perrigo employees worldwide, I am extremely proud to report our tenth consecutive year of record earnings. By effectively executing our ‘Base Plus Plus Plus’ strategy, we continued our consistent track record of delivering superior shareholder returns, with a total shareholder return of over 970% since 2007. Joseph C. Papa Chairman, President, and Chief Executive Officer Over the past eight years, we have transformed Perrigo I also want to address the recent attempts by Mylan N.V. into a top five global OTC pharmaceutical company. (Mylan) to acquire Perrigo. As we have consistently In fiscal 2015, we took significant steps to build on this maintained since April, we believe Mylan’s offer platform through three highly strategic acquisitions that substantially undervalues Perrigo and is not in the best further strengthened our global scale and distribution interests of our shareholders. -

Top 50 Companies Article Pack a Year of Surprises Shakes up Industry Top 10 Sandoz, Mylan and Teva Switch Places at the Top
Top 50 Companies Article Pack A Year Of Surprises Shakes Up Industry Top 10 Sandoz, Mylan And Teva Switch Places At The Top Executive Summary but has vaulted to the top of our sales rankings, In an eventful year for the off-patent industry, the with its $9.37bn total for the combined generics, world’s leading generics and biosimilars players biosimilars, active pharmaceutical ingredients and have switched places in Generics Bulletin’s newly- OTC category narrowly exceeding that of Mylan, compiled global sales ranking. despite the Novartis unit seeing a 1% drop in turnover in 2019. Mylan Prepares For Viatris Merger After a year that has seen major changes for some While Mylan’s total company sales in 2019 grew by of the biggest players in the global off-patent 1% to $11.5bn, our ranking excludes from the key industry, it is perhaps no surprise that Generics category around $1.8bn of Mylan’s turnover from Bulletin’s annual ranking of the world’s top 50 respiratory and allergy brands such as EpiPen generics and biosimilars companies has reflected (epinephrine), Perforomist (formoterol) and Tobi this upheaval. (tobramycin). This year, our annual ranking of the top 50 Mylan was also a firm with very different generics and biosimilars companies is being expectations hanging over it midway through presented in three parts, and our initial look at the last year, with investors keenly anticipating the top 10 companies paints a picture that looks quite firm’s long-trailed management announcement different to the predictions that may have been of restructuring plans expected to revitalize made by industry onlookers just 12 months ago. -

20210316180937477 20-Xxxx
No. 20- IN THE Supreme Court of the United States ABBVIE INC., ABBOTT LABORATORIES, UNIMED PHARMACEUTICALS LLC, AND BESINS HEALTHCARE, INC., Petitioners, v. FEDERAL TRADE COMMISSION, Respondent. ON PETITION FOR A WRIT OF CERTIORARI TO THE UNITED STATES COURT OF APPEALS FOR THE THIRD CIRCUIT PETITION FOR A WRIT OF CERTIORARI WILLIAM F. LEE SETH P. WAXMAN WILMER CUTLER PICKERING Counsel of Record HALE AND DORR LLP LEON B. GREENFIELD 60 State Street CATHERINE M.A. CARROLL Boston, MA 02109 BRITTANY BLUEITT AMADI CLAIRE H. CHUNG JEFFREY I. WEINBERGER MEDHA GARGEYA STUART N. SENATOR WILMER CUTLER PICKERING ADAM R. LAWTON HALE AND DORR LLP MUNGER, TOLLES & OLSON LLP 1875 Pennsylvania Ave., NW 350 South Grand Avenue Washington, DC 20006 Los Angeles, CA 90071 (202) 663-6000 [email protected] ELAINE J. GOLDENBERG MUNGER, TOLLES & OLSON LLP 601 Massachusetts Ave., NW Suite 500 East Washington, DC 20001 Counsel for AbbVie Inc., Abbott Laboratories, and Unimed Pharmaceuticals LLC MELINDA F. LEVITT GREGORY E. NEPPL FOLEY & LARDNER, LLP 3000 K Street, NW Washington, DC 20007 Counsel for Besins Healthcare, Inc. QUESTION PRESENTED Whether the subjective element of the “sham liti- gation” exception to Noerr-Pennington immunity may be met by an inference from a finding that a challenged lawsuit was objectively baseless, even without evidence that the antitrust defendant actually believed the suit lacked merit or was indifferent to the outcome. (i) PARTIES TO THE PROCEEDING Petitioners are AbbVie Inc., Abbott Laboratories, Unimed Pharmaceuticals LLC, and Besins Healthcare, Inc., which were defendants in the district court and appellees and cross-appellants in the court of appeals. -

Horizon Therapeutics Plc
Investor Presentation Horizon Therapeutics plc November 2019 HorizonTherapeutics.com Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements related to Horizon’s full-year 2019 net sales and adjusted EBITDA guidance; expected financial performance and operating results in future periods, including potential growth in net sales of certain of Horizon’s medicines; expected impact of refinancing transactions; expected timing of clinical trials and regulatory submissions and decisions, including related to the Biologics License Application (BLA) for teprotumumab; potential market opportunity for and benefits of Horizon’s medicines and medicine candidates; and business and other statements that are not historical facts. These forward-looking statements are based on Horizon’s current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation, risks that Horizon’s actual future financial and operating results may differ from its expectations or goals; Horizon’s ability to grow net sales from existing medicines; the availability of coverage and adequate reimbursement and pricing from government and third-party payers; risks relating to Horizon’s ability to successfully implement its business strategies; risks inherent in developing novel medicine candidates, such as -

ANALYSIS of AGREEMENT CONTAINING CONSENT ORDERS to AID PUBLIC COMMENT in the Matter of Mylan N.V., File No
ANALYSIS OF AGREEMENT CONTAINING CONSENT ORDERS TO AID PUBLIC COMMENT In the Matter of Mylan N.V., File No. 151-0129 The Federal Trade Commission (“Commission”) has accepted, subject to final approval, an Agreement Containing Consent Orders (“Consent Agreement”) from Mylan N.V. (“Mylan”) that is designed to remedy the anticompetitive effects resulting from Mylan’s acquisition of Perrigo Company plc (“Perrigo”). Under the terms of the proposed Consent Agreement, Mylan is required to divest to Alvogen, Inc. (“Alvogen”) all of its rights and assets to the following generic pharmaceutical products: (1) acyclovir ointment; (2) bromocriptine mesylate tablets; (3) clindamycin phosphate/benzoyl peroxide gel; (4) hydromorphone hydrochloride extended release tablets; (5) liothyronine sodium tablets; (6) polyethylene glycol 3350 over-the-counter (“OTC”) oral solution packets; and (7) scopolamine extended release transdermal patches. The proposed Consent Agreement has been placed on the public record for thirty days for receipt of comments from interested persons. Comments received during this period will become part of the public record. After thirty days, the Commission will again evaluate the proposed Consent Agreement, along with the comments received, to make a final decision as to whether it should withdraw from the proposed Consent Agreement or make final the Decision and Order (“Order”). On September 14, 2015, Mylan launched a hostile tender offer to gain a controlling interest in Perrigo. The Commission alleges in its Complaint that the proposed acquisition, if consummated, would violate Section 7 of the Clayton Act, as amended, 15 U.S.C. § 18, and Section 5 of the Federal Trade Commission Act, as amended, 15 U.S.C. -
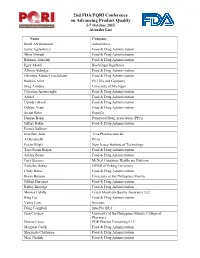
2Nd FDA/PQRI Conference on Advancing Product Quality 5-7 October 2015 Attendee List
2nd FDA/PQRI Conference on Advancing Product Quality 5-7 October 2015 Attendee List Name Company Bertil Abrahamsson AstraZeneca Jenny Agila-Sefen Food & Drug Administration Mina Ahmadi Food & Drug Administration Behnam Alizadeh Food & Drug Administration Ilgaz Akseli Boehringer Ingelheim Allison Aldridge Food & Drug Administration Christina Alemu-Cruickshank Food & Drug Administration Barbara Allen Eli Lilly and Company Greg Amidon University of Michigan Celestina Arowosegbe Food & Drug Administration Ashraf Food & Drug Administration Upinder Atwal Food & Drug Administration Debbie Avant Food & Drug Administration Stefan Baier PepsiCo Denyse Baker Parenteral Drug Association (PDA) Jeffrey Baker Food & Drug Administration Patrick Ballmer Inna Ben-Anat Teva Pharmaceuticals Al Berchielli Pfizer Ecevit Bilgili New Jersey Institute of Technology Tara Gooen Bizjak Food & Drug Administration Ashley Boam Food & Drug Administration Gary Boerner McNeil Consumer Healthcare Division Nicholas Buhay CPIER of Peking University Cindy Buhse Food & Drug Administration Bryan Bulatao University of the Philippines Manila Gilbert Burckart Food & Drug Administration Kelley Burridge Food & Drug Administration Monica Cahilly Green Mountain Quality Assurance LLC Bing Cai Food & Drug Administration Yanxi Cain Novartis Doug Campbell InterPro QRA Jean Casauay University of the Philippines Manila, College of Pharmacy Dennis Casey PDR Pharma Consulting LLC Margaret Caulk Food & Drug Administration Sharmista Chatterjee Food & Drug Administration Mari Cheliah Food &