Mammalian Molar Complexity Follows Simple, Predictable Patterns
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tooth Size Proportions Useful in Early Diagnosis
#63 Ortho-Tain, Inc. 1-800-541-6612 Tooth Size Proportions Useful In Early Diagnosis As the permanent incisors begin to erupt starting with the lower central, it becomes helpful to predict the sizes of the other upper and lower adult incisors to determine the required space necessary for straightness. Although there are variations in the mesio-distal widths of the teeth in any individual when proportions are used, the sizes of the unerupted permanent teeth can at least be fairly accurately pre-determined from the mesio-distal measurements obtained from the measurements of already erupted permanent teeth. As the mandibular permanent central breaks tissue, a mesio-distal measurement of the tooth is taken. The size of the lower adult lateral is obtained by adding 0.5 mm.. to the lower central size (see a). (a) Width of lower lateral = m-d width of lower central + 0.5 mm. The sizes of the upper incisors then become important as well. The upper permanent central is 3.25 mm.. wider than the lower central (see b). (b) Size of upper central = m-d width of lower central + 3.25 mm. The size of the upper lateral is 2.0 mm. smaller mesio-distally than the maxillary central (see c), and 1.25 mm. larger than the lower central (see d). (c) Size of upper lateral = m-d width of upper central - 2.0 mm. (d) Size of upper lateral = m-d width of lower central + 1.25 mm. The combined mesio-distal widths of the lower four adult incisors are four times the width of the mandibular central plus 1.0 mm. -

Third Molar (Wisdom) Teeth
Third molar (wisdom) teeth This information leaflet is for patients who may need to have their third molar (wisdom) teeth removed. It explains why they may need to be removed, what is involved and any risks or complications that there may be. Please take the opportunity to read this leaflet before seeing the surgeon for consultation. The surgeon will explain what treatment is required for you and how these issues may affect you. They will also answer any of your questions. What are wisdom teeth? Third molar (wisdom) teeth are the last teeth to erupt into the mouth. People will normally develop four wisdom teeth: two on each side of the mouth, one on the bottom jaw and one on the top jaw. These would normally erupt between the ages of 18-24 years. Some people can develop less than four wisdom teeth and, occasionally, others can develop more than four. A wisdom tooth can fail to erupt properly into the mouth and can become stuck, either under the gum, or as it pushes through the gum – this is referred to as an impacted wisdom tooth. Sometimes the wisdom tooth will not become impacted and will erupt and function normally. Both impacted and non-impacted wisdom teeth can cause problems for people. Some of these problems can cause symptoms such as pain & swelling, however other wisdom teeth may have no symptoms at all but will still cause problems in the mouth. People often develop problems soon after their wisdom teeth erupt but others may not cause problems until later on in life. -

Unusual Anatomy of a Second Maxillary Molar - a Rare Four- Root Configuration Case Report
ARC Journal of Dental Science Volume 1, Issue 2, 2016, PP 13-15 ISSN No. (Online): 2456-0030 http://dx.doi.org/10.20431/2456-0030.0102003 www.arcjournals.org Unusual Anatomy of a Second Maxillary Molar - a Rare four- Root Configuration Case Report Dr. Thiago de Almeida Prado Naves Carneiro,DDS, MSc PhD student, Department of Occlusion, Fixed Prostheses, and Dental Materials, School of Dentistry, Universidade Federal de Uberlândia, Uberlândia, Minas Gerais Brazil. [email protected] Abstract: Although it is a very rare situation, four-rooted maxillary second molars can occur. The existence of two palatal roots is extremely rare and ranges about only 0.4%. The aim of this study is to present and document a very rare anatomic configuration of a four-rooted maxillary second molar. Anatomic variation in the number of roots and root canals can occur in any tooth, although some cases can be extremely rare as the one presented here.Clinicians should be aware of this possibility before considering any kind of treatment. Keywords: Molar, Dental Anatomy, Anatomical Variation 1. INTRODUCTION Usually the maxillary second molars are described in the literature as a teeth that have 3 roots with 3 or 4 root canals. Understanding of the presence of additional roots and unusual root canals is essential and determines the success of endodontic treatment1. The existence of maxillary second molars with 4 roots (2 buccal and 2 palatal) is extremely rare and ranges about only 0.4%.This information comes from a study that showed, after the examination of two different horizontally angled radiographs of 1,000 maxillary second molars, just four with four roots2. -

Two Sets of Teeth in a Lifetime
Two sets of teeth in a lifetime Two sets of teeth in a lifetime Deciduous teeth: They are the first set of teeth we have and there are altogether 20 of them. They usually start to erupt from around the age of six months until 3 years of age. Permanent teeth: At the age of 6, they sequentially erupt to replace the deciduous teeth which become loose and shed. Deciduous teeth: Space retainer for permanent teeth Normally, underneath the root of each deciduous tooth, there is a developing permanent successor tooth. When it is time for the permanent successor tooth to erupt, the root of the deciduous tooth will resorb and the deciduous tooth will become loose. The place is then taken up by its permanent successor tooth. Deciduous tooth retains the space for its permanent successor tooth. No tooth is dispensable If the deciduous tooth, especially the second deciduous molar, is lost early due to tooth decay, the consequences can be serious: Poor alignment of the teeth The second deciduous molar is already lost The first permanent molar Since the first permanent molar erupts behind the second deciduous molar at the age of 6, the space of the lost second deciduous molar will gradually close up as the first permanent molar moves forward. The permanent tooth is crowded out of the arch when it erupts Later, when the second permanent premolar erupts to replace the second deciduous molar, the permanent tooth will either be crowded out of the dental arch or be impacted and is unable to erupt, leading to poor alignment of the teeth. -

A Comparative Study of One Implant Versus Two Replacing a Single Molar Thomas J
JOMI on CD-ROM, 1996 Mar (372-378 ): A Comparative Study of One Implant Versus … Copyrights © 1997 Quinte… A Comparative Study of One Implant Versus Two Replacing a Single Molar Thomas J. Balshi, DDS, FACP/Ramon E. Hernandez, DMD/Maria Claudia Pryszlak, DMD/Bo Rangert, PhD, MechEng A comparative study between one and two Brånemark implants replacing a single molar was conducted. Forty-seven individuals comprised two groups of 22 patients treated with one implant and 25 with two implants. A total of 72 implants were placed, 66 (92%) in the mandible and six (8%) in the maxilla. After the first year of function, the success rate was 99%, with only one implant lost. Between the second- and third-year follow-ups, 100% of the implants continued to function in the remaining 46 patients, giving a 3-year cumulative success rate of 99%. The marginal bone loss between 1 and 3 years of function was 0.10 mm (SD 0.20) for the group with one implant and 0.24 mm (SD 0.20) for the group with two implants. No changes were observed in the Sulcus Bleeding Index during the 3-year follow-up. Prosthesis mobility or screw loosening was the most frequent complication and was predominant in the group using one implant (48%), but was substantially reduced in the group using two implants (8%). These mechanical problems, using one implant only, seem to be preventable using a stronger screw joint (CeraOne abutment). Precise centric occlusal contact was established and maintained over the study period, which was thought to contribute to the very high success rate for the single-implant-supported molars, despite their high degree of mechanical problems. -

CHAPTER 5Morphology of Permanent Molars
CHAPTER Morphology of Permanent Molars Topics5 covered within the four sections of this chapter B. Type traits of maxillary molars from the lingual include the following: view I. Overview of molars C. Type traits of maxillary molars from the A. General description of molars proximal views B. Functions of molars D. Type traits of maxillary molars from the C. Class traits for molars occlusal view D. Arch traits that differentiate maxillary from IV. Maxillary and mandibular third molar type traits mandibular molars A. Type traits of all third molars (different from II. Type traits that differentiate mandibular second first and second molars) molars from mandibular first molars B. Size and shape of third molars A. Type traits of mandibular molars from the buc- C. Similarities and differences of third molar cal view crowns compared with first and second molars B. Type traits of mandibular molars from the in the same arch lingual view D. Similarities and differences of third molar roots C. Type traits of mandibular molars from the compared with first and second molars in the proximal views same arch D. Type traits of mandibular molars from the V. Interesting variations and ethnic differences in occlusal view molars III. Type traits that differentiate maxillary second molars from maxillary first molars A. Type traits of the maxillary first and second molars from the buccal view hroughout this chapter, “Appendix” followed Also, remember that statistics obtained from by a number and letter (e.g., Appendix 7a) is Dr. Woelfel’s original research on teeth have been used used within the text to denote reference to to draw conclusions throughout this chapter and are the page (number 7) and item (letter a) being referenced with superscript letters like this (dataA) that Treferred to on that appendix page. -
Identifying Features
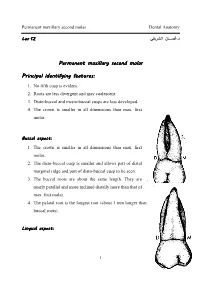
Permanent maxillary second molar Dental Anatomy .د ـــن ا Lec.Lec.12121212 Permanent maxillary second molar Principal identifying features: 1. No fifth cusp is evident. 2. Roots are less divergent and may coalescent. 3. Disto-buccal and mesio-buccal cusps are less developed. 4. The crown is smaller in all dimensions than max. first molar. Buccal aspect: 1. The crown is smaller in all dimensions than max. first molar. 2. The disto-buccal cusp is smaller and allows part of distal marginal ridge and part of disto-buccal cusp to be seen. 3. The buccal roots are about the same length. They are nearly parallel and more inclined distally more than that of max. first molar. 4. The palatal root is the longest root (about 1 mm longer than buccal roots). Lingual aspect: 1 Permanent maxillary second molar Dental Anatomy 1. The disto-lingual cusp is smaller than that of max. first molar. 2. No fifth cusp. 3. The apex of palatal root is in a line with disto-lingual cusp tip . Mesial aspect: 1. Bucco-lingual dimension is the same as that of max. first molar but the crown length is smaller. 2. The roots are less divergent bucco-lingually. Distal aspect: The disto-buccal cusp is smaller than that of max. first molar. Occlusal aspect: 1. The crown has rhomboidal shape, with the acute angles are less and the obtuse angles are more than that of max. first molar. 2. The bucco-lingual diameter is the same but the mesio-distal diameter is smaller by about 1 mm than that of max. -

Congenitally Missing Mandibular Premolars — Treatment Options for Space Closure
CONTINUING EDUCATION Congenitally missing mandibular premolars — treatment options for space closure Dr. Mark W. McDonough discusses recognition and treatment planning for congenitally missing second premolars Introduction The orthodontist often identifies missing Educational aims and objectives second premolars in the mixed dentition This article aims to direct the orthodontist through a diagnostic sequence of recognizing and treatment planning for congenitally missing second premolars. using routine panoramic radiographs. The early decisions that orthodontists make for Expected outcomes the congenitally missing teeth often have an Orthodontic Practice US subscribers can answer the CE questions on page 22 to impact on dental health for the rest of their earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can: patient’s life. Therefore, this finding should • Realize some diagnoses of missing mandibular premolars. result in a comprehensive set of diagnostic • Realize some treatment options for mandibular premolars. records in order to evaluate the patient in all three planes of space and establish a • Identify critical factors to consider to avoid complications. problem list and treatment alternatives. • Identify three different methods of space closure from the case studies presented. These records often need to be shared with the restorative dentist and other specialists in order to consider all viable alternatives and formulate a proper treatment plan. General concepts about missing -

Molar-Incisor Hypomineralisation (MIH)
Further resources British Society of Paediatric Dentistry Position Statement: https://www.bspd.co.uk/Portals/0/MIH%20statement%20final%20J an%202020.pdf Department of Health Preventive Guidelines: https://assets.publishing.service.gov.uk/government/uploads/syste m/uploads/attachment_data/file/601833/delivering_better_oral_hea lth_summary.pdf Photo is courtesy of the University of Oslo, Department of Paediatric Dentistry. Licenced under CC BY 4.0 Patient advice and liaison service (PALS) Molar-Incisor If you have a compliment, complaint or concern please contact our Hypomineralisation (MIH) PALS team on 020 7288 5551 or [email protected] If you need a large print, audio or translated copy of this leaflet A Patient’s Guide please contact us on 020 7288 3182. We will try our best to meet your needs. Twitter.com/WhitHealth Facebook.com/WhittingtonHealth Whittington Health NHS Trust Magdala Avenue London N19 5NF Phone: 020 7272 3070 www.whittington.nhs.uk Date published: 25/06/2020 Review date: 25/06/2022 Ref: SC/DentServ/MIH/01 © Whittington Health Please recycle What is MIH? How is MIH treated? MIH is a dental condition which affects the outer layer of a Your dentist will help you chose the best option for your child tooth, the enamel. In MIH, the enamel is softer than usual and depending on their age, co-operation and severity of the defect. therefore these teeth are more prone to sensitivity and decay. Treatment is aimed at reducing sensitivity and preventing The adult molars (back teeth) and incisors (front teeth) are the further tooth breakdown. For more severely affected teeth, most commonly affected teeth. -

Dual Origin of Tribosphenic Mammals
articles Dual origin of tribosphenic mammals Zhe-Xi Luo*, Richard L. Cifelli² & Zo®a Kielan-Jaworowska³ *Section of Vertebrate Paleontology, Carnegie Museum of Natural History, Pittsburgh, Pennsylvania 15213, USA ² Oklahoma Museum of Natural History, 2401 Chautauqua, Norman, Oklahoma 73072, USA ³ Institute of Paleobiology, Polish Academy of Sciences, ulica Twarda 51/55, PL-00-818 Warszawa, Poland ............................................................................................................................................................................................................................................................................ Marsupials, placentals and their close therian relatives possess complex (tribosphenic) molars that are capable of versatile occlusal functions. This functional complex is widely thought to be a key to the early diversi®cation and evolutionary success of extant therians and their close relatives (tribosphenidans). Long thought to have arisen on northern continents, tribosphenic mammals have recently been reported from southern landmasses. The great age and advanced morphology of these new mammals has led to the alternative suggestion of a Gondwanan origin for the group. Implicit in both biogeographic hypotheses is the assumption that tribosphenic molars evolved only once in mammalian evolutionary history. Phylogenetic and morphometric analyses including these newly discovered taxa suggest a different interpretation: that mammals with tribosphenic molars are not monophyletic. Tribosphenic -

Tooth Anatomy
Tooth Anatomy To understand some of the concepts and terms that we use in discussing dental conditions, it is helpful to have a picture of what these terms represent. This picture is from the American Veterinary Dental College. Pulp Dentin Crown Enamel Gingiva Root Periodontal Ligament Alveolar Bone supporting the tooth Crown: The portion of the tooth projecting from the gums. It is covered by a layer of enamel, though dentin makes up the bulk of the tooth structure. The crown is the working part of the tooth. In dogs and cats, most teeth are conical or pyramidal in shape for cutting and shearing action. Gingiva: The gum tissue surrounds the crown of the tooth and protects the root and bone which are subgingival (below the gum line). The gingiva is the first line of defense against periodontal disease. The space where the gingiva meets the crown is where periodontal pockets develop. Measurements are taken here with a periodontal probe to assess the stage of periodontal disease. When periodontal disease progresses it can involve the Alveolar Bone, leading to bone loss and root exposure. Root Canal: The root canal contains the pulp. This living tissue is protected by the crown and contains blood vessels, nerves and specialized cells that produce dentin. Dentin is produced throughout the life of the tooth, which causes the pulp canal to narrow as pets age. Damage to the pulp causes endodontic disease which is painful, and can lead to infection and loss of the tooth. Periodontal Ligament: This tissue is what connects the tooth root to the bone to keep it anchored to its socket. -

Management of Molar Incisor Hypomineralization: Foundational Articles and Consensus Recommendations
Management of Molar Incisor Hypomineralization: Foundational Articles and Consensus Recommendations. 2020 Da Cunha Coelho A, Mata P, Lino CA, Macho VP, Areias C, Norton A, Augusto A. Dental hypomineralization treatment: A systematic review. J Esthet Restor Dent. 2019; 31: 26-39. Elhennawy K, Schwendicke F. Managing molar-incisor hypomineralization: A systematic review. J of Dent 2016; 55: 16-24. Elhennawy K, Manton DJ, Crombie F, Zaslansky P, Radlanski RJ, et al. Structural, mechanical and chemical evaluation of molar-incisor hypomineralization-affected enamel: A systematic review. Arch Oral Biol 2017; 83: 272-281. Elhennawy K, Jost-Brinkmanna PG, Manton DJ, Paris S, Schwendicke F. Managing molars with severe molar- incisor hypomineralization: A cost-effectiveness analysis within German healthcare. J Dent 2017; 63: 65-71. Garot E, Denis A, Delbos Y, Manton D, Silva M, Rouas P. Are hypomineralised lesions on second primary molars (HSPM) a predictive sign of molar incisor hypomineralisation (MIH)? A systematic review and a meta- analysis. J Dent. 2018; 72: 8-13 Ghanim A, Silva MJ, Elfrink MEC, Lygidakis NA, et al. Molar incisor hypomineralisation (MIH) training manual for clinical field surveys and practice. Eur Arch Paediatr Dent 2017; 18(4): 225-242. Gulser K, Cetin M, Bose B, Ellidokuz H. Prevalence, aetiology and treatment of molar incisor hypomineralization in children living in Izmir City (Turkey). Int J Paediatr Dent 2019; 29: 775-782. Jälevik B, Möller M. Evaluation of spontaneous space closure and development of permanent dentition after extraction of hypomineralized permanent first molars. Int J Paediatr Dent. 2007;17: 328-35. Lygidakis NA, Wong F, Jälevik B, Vierrou AM, et al.