Nemoralis Shell Polymorphism in the Pyrenees Over Five Decades
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NFBB Vol. 21 1946
Issued Quarterly by the Western Bird-Banding Association The Editors Resign New Members: A Bigger and Better W.B.B.A. Banded Gulls Pioneer OI~ithologist - Joseph Mailliard Results from Wildlife Service Files of Banded Robins Band-tailed Pigeons Nest in Colorudo Bandlng Activities with Birds of Prey in 1944 and 1945 Dea.d Owls and Mou.rning Doves Not How Many but How Rare California Blue Grosbeaks at Pomona Annual Report - 1945 Banders Who Reported for the Year 1945 Notes on Colorado Birds Banders at the Mission Nest Parasites Band Records Lost Traps Made Available Co-editors of the ~ President of W.E.B.A --- F. G. Crawford Mary M. Erickson, Santa Barbare'J Calif. 2782 Glen Avenue, Altadena, California Frank M. Erickson, Santa Barbara, Calif. Address all contributions to the ~ to Mrs. M. C. Sargent, P. O. Box 109, La Jolla, California At the request of P~esident F. G. Crawfo~d and with due modesty, the Editors give place to the following statement: "At its January meeting, the councillo!'s of W.E.B.A. extended to me the privilege of voicing their gratitude for the faithful and excellent editorial work that Dr. Mary M. Erickson and Dean Frank M. Erickson have devoted to the ~ for the past five years. It is certain that the entire membership of the Association joins the Council in this appreciution. We deeply regret that the co-editors find it necessary to conclude their editorial duties with this issue. "That the News hus continued to grow in quality is solely due to the efforts of our co-editors. -

Dear Parents/Carers, F2 Lilac Pink Red Year 1 Yellow Blue Green
Dear Parents/Carers, Over the next few weeks, we will be making changes to the system we use to grade the reading books that the children bring home. All books Lilac will be placed in a colour band and will no longer be given a number or F2 Pink stage. The same system will be used throughout the school, making it Red easier to track the children's progress. Yellow Blue Over the next week or two, we will be assessing each child’s reading level. Year 1 Green In the past, the emphasis has been on decoding skills. However, we will Orange now also be looking at their comprehension, because reading success Turquoise depends not only on being able to read the words but also on their Purple understanding. This also means that children need to use the punctuation Gold to make sense of the text. When we assess the children, we will be Year 2 White analysing their errors to understand what their next steps are. Obviously, this will take some time for us to get to grips with, so we hope that you Year 3 will be understanding while we get round every child. You might feel that Lime your child has ‘gone down a level’. Please be assured that this is not the Year 4 case, because we are also looking at comprehension. Brown Year 5 The colour band system begins with lilac books. These books have no Grey words and will be given to those children who are getting ready to read. Dark Blue Lilac books can be used to teach a child how books work and to encourage Year 6 Burgundy them to tell a story using the pictures. -

P0395-P0400.Pdf
TheCondor95:395-400 0 The Cooper Omthological Soaety 1993 AN EXPERIMENTAL TEST OF THE CONTRASTING-COLOR HYPOTHESIS OF RED-BAND EFFECTS IN RED-WINGED BLACKBIRDS ’ KAREN J. METZ AND PATRICK J. WEATHERHEAD Department of Biology, Carleton University,Ottawa, Ontario KlS 5B6, Canada Abstract. We performed an experiment to investigate effects of red leg bands on the behavior of male Red-winged Blackbirds(Agelaiusphoeniceus). We presentedmodels having either black, blue, or red leg bands to territorial male Red-winged Blackbirds to test the hypothesisthat any color of band that contrastswith the color of the legsmakes an individual appear abnormal or unhealthy and therefore subject to attack. Overall, territorial males respondedequally aggressivelyto the black- and blue-banded models, but spent more time at distancesgreater than 10 m, displayedat lower intensities, and took longer to attack when the model was given red bands. Thus, red bands appearedto make the model initially more threatening to territorial male Red-winged Blackbirds. These results do not support the contrasting-color hypothesis and suggestthat the effect of red bands is attributable to the bands matching the color of the male’s epaulets. The failure of the contrasting-color hy- pothesisalso leaves unresolvedthe different outcomesof one previous experiment showing a negative effect of red bands, and two analysesof long-term banding studiesthat detected no effect of red bands. Key words: Red-wingedBlackbird; Agelaius phoeniceus;epaulets; colored bands. INTRODUCTION than red-banded males with smaller epaulets We recently demonstrated that red leg bands se- (Metz and Weatherhead 199 1). The causeof the verely reduced the ability of male Red-winged territory loss appeared to be increased and more Blackbirds(Ageluiusphocnicrus) to maintain their persistent intrusion by other males, particularly territories, unlike black bands which had no no- territorial neighbors. -

Color Change in Hughes's Celluloid Leg Bands
j. Field Ornithol., 66(2):289-295 COLOR CHANGE IN HUGHES'S CELLULOID LEG BANDS GERALD D. LINDSEY, KAREN A. WILSON AND CHRISTINA HERRMANN National BiologicalSurvey Alaska Science Center Hawaii Field Station P.O. Box 44 Hawaii National Park, Hawaii 96718 USA Abstract.--Colorchange was recorded in 10 colorsfor 237 Hughescelluloid bands attached to the tarsi of 84 birds for up to 5 yr and for 45 celluloid bands exposedto natural sunlight on Mauna Kea, Hawaii. Colors of four solid-coloredbands (mauve, light green, yellow and black) and two split-coloredbands (orange/dark green and dark blue/white) attached to free-flyingbirds were still recognizable->3 yr. Within 2-3 yr, light blue, orange and red solid- colored bands and a red/white split-coloredband had faded and could be confusedwith other colors.For studies--<2 yr, most Hughes celluloid bands retained enough color to be recognizedunder field conditions.For long-termstudies, biologists should select only those colorsthat can be recognizedfor the duration of the studyor useanother type of color band that is more resistantto fading or discoloration. CAMBIO EN COLORACION DE BANDAS HUGHES Sinopsis.--Seencontr6 cambio en la coloraci6nde 237 bandasplfisticas Hughes de 10 colores diferentesque estuvieroncolocadas pot cinco aftosen el tarsode 84 pfijarosy de 45 bandas expuestasa la luz natural en Mauna Kea, Hawaii. Bandasde cuatro coloress61idos y de dos coloresmixtos pudieron ser reconocidasluego de haber estadopor m'fisde 3 aftos en las patas de avessilvestres. No obstante, entre 2-3 aftos,las bandas de colores s61idoscomo el azul p'filido,anaranjado, rojo y bandasde coloresmixtos como el rojo/blanco hab/an cam- biado hastael punto de poder set confundidascon otros colores.Para estudios<2 aftos,las bandasHughes retienen el suficientecolor para poder set reconocidasen traba.jos de campo. -

Effects of Different Marking Techniques on the Fitness of Avian Species
Effects of different marking techniques on the fitness of avian species. Abstract In science it is of great importance to differentiate individuals in certain experiments on animals. There are many marking techniques for identification of these individuals, and before, scientists did not assume that these techniques would interfere with their results. However, recent studies have shown that markings may affect certain aspects of an animal’s fitness. Therefore, in this literature study, I examine the possible effects different markings may have on the fitness of avian species. I found that there are some physical effects, affecting the animal’s reproductive success and mortality. Many effects due to certain markings techniques may influence one another. In some of the cases, we can differentiate between effects on mortality and reproductive success, but it is difficult to specify the cause of these effects. There was also substantial impact found of different colors of markings, mainly on sexual communication and communication with competitors, thereby affecting reproductive success of birds. Findings lead to the hypothesis that colors naturally occurring on a birds plumage or soft parts is perceived as an exaggeration of an attractive trait, so therefore perceived as more attractive, unless the concerning color is present in a congener’s plumage or soft parts, indicating the possibility that color preference may play its role in the evolution of species recognition. It should be acknowledged that marking techniques may indeed have effects in certain avian species, and may therefore compromise the accuracy of acquired data. To minimize physical effects, the marking should be of proper size, suitable for the species of interest. -

Guidelines to the Use of Wild Birds in Research
THE ORNITHOLOGICAL COUNCIL Providing Scientific Information about Birds GUIDELINES TO THE USE OF WILD BIRDS IN RESEARCH Special Publication 1997 Edited by Abbot S. Gaunt & Lewis W. Oring Third Edition 2010 Edited by Jeanne M. Fair, Editor-in-Chief Ellen Paul & Jason Jones, Associate Editors GUIDELINES TO THE USE OF WILD BIRDS IN RESEARCH Jeanne M. Fair1, Ellen Paul2, & Jason Jones3, Anne Barrett Clark4, Clara Davie4, Gary Kaiser5 1 Los Alamos National Laboratory, Atmospheric, Climate and Environmental Dynamics, MS J495, Los Alamos, NM 87506 2 Ornithological Council, 1107 17th St., N.W., Suite 250, Washington, D.C. 20036 3 Tetra Tech EC, 133 Federal Street, 6th floor, Boston, Massachusetts 02110 4 Binghamton University State University of New York, Department of Biology, PO BOX 6000 Binghamton, NY 13902-6000 5 402-3255 Glasgow Ave, Victoria, BC V8X 4S4, Canada Copyright 1997, 2010 by THE ORNITHOLOGICAL COUNCIL 1107 17th Street, N.W. Suite 250 Washington, D.C. 20036 http://www.nmnh.si.edu/BIRDNET Suggested citation Fair, J., E. Paul, and J. Jones, Eds. 2010. Guidelines to the Use of Wild Birds in Research. Washington, D.C.: Ornithological Council. Revision date August 2010 2 Dedication The Ornithological Council dedicates this 2010 revision to Lewis W. Oring and the late Abbot (Toby) S. Gaunt, whose commitment to the well-being of the birds for whom ornithologists share a deep and abiding concern has served our profession well for so many years. Toby Gaunt Lew Oring Revision date August 2010 3 Acknowledgments and disclaimer Third edition The Ornithological Council thanks the Office of Laboratory Animal Welfare of the National Institutes of Health for their financial support for the production of this revision. -

Orbitally Forced Sphalerite Growth in the Upper Mississippi Valley District
© 2019 The Authors Published by the European Association of Geochemistry Orbitally forced sphalerite growth in the Upper Mississippi Valley District M. Li1, H.L. Barnes1* Abstract doi: 10.7185/geochemlet.1929 Groundwater plays an important role in global water cycles and Earth’s climate system. Nevertheless, the geologic history of groundwater activity remains unclear due to limited data. Sphalerite colour banding in the Upper Mississippi Valley District (USA) is appar- ently caused by variation in oxidation state during precipitation which is controlled by the penetration of deeply circulating groundwater. Here, time series analysis of the grayscale profile of the Permian sphalerite banding in this district shows the banding correlates with Earth’s eccentricity, obliquity, and precession forcing. We have found that the astro- nomical forcing paced the fluxes of heat and precipitation and regulated the penetration of groundwater into the ore zone and thus the sphalerite banding. The results demonstrate that banding in sphalerite follows the Milankovitch climate frequencies over 104 – 105 years. Therefore, we show that groundwater has a major role in depositing the iron-rich bands of the sphalerite and, as a final corollary, that the banding itself can be used to decipher the effects of climate on groundwater variations in the global water cycle. Received 19 June 2019 | Accepted 5 October 2019 | Published 12 November 2019 Introduction was about 1 km, based on palaeostratigraphic reconstruc- tion (Rowan and Goldhaber, 1996). In essence, the banding Mississippi Valley Type deposits yield about 24% of the global recorded variability in geochemical conditions from ground- commercial zinc and lead resources (Leach et al., 2010). -

Effects of Band Color on Survivorship, Body Condition and Reproductive Effort of Free-Living Australian Zebra Finches
The Auk 111(1):131-142, 1994 EFFECTS OF BAND COLOR ON SURVIVORSHIP, BODY CONDITION AND REPRODUCTIVE EFFORT OF FREE-LIVING AUSTRALIAN ZEBRA FINCHES RICHARD ZANN Departmentof Zoology,La TrobeUniversity, Bundoora, Victoria, 3083 Australia ABSTRACT.--Indomesticated laboratory Zebra Finches (Taeniopygiaguttata), leg bands of certaincolors affect mate selection,parental effort, reproductivesuccess, sex ratio of offspring, and survivorship.I tested the effectsof these same colorson survivorship,body condition and reproductiveeffort of free-living Zebra Finchesover severalbreeding seasonsat two coloniesin northern Victoria,Australia. At the "Cloverlea" colony,adults of eachsex received either "attractive"or "unattractive"color bands.Nevertheless, survivorship to 12 months was not affectedby band color nor was there compellingevidence (mass, bill color and molt) for deterioratingbody conditiondue to reproductivecosts; there were no sexdifferences in survivorship.Reproductive effort eachbreeding season in thesefree-living birds may not be as great as that in some laboratory populationsbecause in nature the seasonis shorter and the intervalfor recoverycorrespondingly longer. In contrast,at the "Danaher"colony, where birds had unique color combinationsof three bands(plus one metal band), a retrospective analysisof four years of data showed that there were significanteffects of band color on reproductive effort of females;they laid more eggs if mated to males having a single red band (attractivecolor) in their combinationthan if mated to maleshaving a single light- green band (unattractive color) or bands of other colors (neutral attractiveness).They laid an extra clutch, but this did not result in significantly more fledglings and independent young being raisedby the pair. There were no band-coloror sex effectson survivorshipof adults at this colony. There were no obvious biases in the allocation of band colors or identificationof breedingpairs that might explainthe extra-clutcheffect. -

Pa Series for Mission Critical Desktop Applications Featuring Pa242w | Pa272w | Pa302w
NEC DISPLAY SOLUTIONS PA SERIES FOR MISSION CRITICAL DESKTOP APPLICATIONS FEATURING PA242W | PA272W | PA302W For Businesses that demand the Highest Performance 10-Bit AH-IPS Panel with Image Uniformity, GB-R LED Backlighting, Next Generation Input Options If you run a creative, design, photographic, architectural or precision engineering business that benefits from accurate imaging, you will need a display that is best suited to intense, concentrated operation cycles in terms of performance and DESIGNED FOR ARCHITECTURE, ENGINEERING, INDUSTRIAL user experience. The new NEC MultiSync PA Series offers the perfect solution. DESIGN, NDT AND PRECISION CAD FIELDS 10-bit AH-IPS Panel delivering the ultimate in image accuracy The selection of best-in-class ergonomics and a 10-bit AH-IPS Panel with GB-R backlighting and 10-bit input via DisplayPort means guaranteed outstanding 1 billion colours with 3D colour emulation look up table image and colour reproduction, but more importantly delivered improved GB-R LED for the latest mercury-free and low energy backlighting productivity. With an ideal visual working environment for your employees and source with wide gamut capability with top monitor quality, an increased initial outlay will be quickly repayed, whilst offering better TCO and comfort. Image Uniformity Control and Backlight Ageing Correction Advanced connectivity including 10 bit DisplayPort input With an initial guarantee and the NEC brand promise of trusted performance in the most demanding applications behind it, making an outlay in the best display PIP combinations, multiple input source, simultaneous viewing in its class is a wise choice for those who value continuity, consistency and 24/7 Warranty Extension precision in their working process. -

Chromosome 2 Aberrations in Clinical Cases Characterised by High
434 LETTER TO JMG Chromosome 2 aberrations in clinical cases characterised by high resolution multicolour banding and region specific FISH probes A Weise, H Starke, A Heller, H Tönnies, M Volleth, M Stumm, S Gabriele, A Nietzel, U Claussen, T Liehr ............................................................................................................................. J Med Genet 2002;39:434–439 he field of human cytogenetics has been through many YAC, BAC, and cosmid probes for chromosomes 2 and 9 were different stages of development, each of them improving used in combination with the MCB probe sets or separately in Tthe characterisation of structurally abnormal and/or one and two colour FISH experiments14; these probes are listed supernumerary chromosomes. The era of reliable identifica- in table 2. Moreover, a probe for all human telomeres (DAKO) tion of human chromosomes started with the invention of the was hybridised on chromosomes of case 11 and a probe banding method by Dr Lore Zech in 1968.1 The introduction of specific for the short arms of all human acrocentric fluorescence in situ hybridisation (FISH) techniques in chromosomes (midi 54, described in Mrasek et al13)was human cytogenetics by Pinkel et al2 in 1986 allowed specific applied in case 4. CGH15 was performed in cases 1, 6, and 7. staining of chromosomes and chromosomal subregions. Even Metaphase spreads were analysed using a fluorescence though G banding3 is still the gold standard against which all microscope (Axioplan 2 mot, Zeiss) equipped with appropriate molecular cytogenetic techniques are measured, this tech- filter sets to discriminate between a maximum of five fluoro- nique based on alternating light and dark bands can lead to chromes and the counterstain DAPI (diaminophenylindol). -

Chroma Reconstruction from Inaccurate Measurements
Chroma Reconstruction from Inaccurate Measurements Alexander Balinsky Nassir Mohammad Cardiff University, UK Cardiff University & HP Labs, UK [email protected] [email protected] ABSTRACT Non-linear filter responses of natural colour images have been shown to display non-Gaussian heavy tailed distributions which we call sparse. These filters operate in the YUV colour space on the chroma channel U (and V) using weighting functions obtained from the gray image Y. In this paper we utilise this knowledge for denoising the chroma channels of a colour image from inaccurate measurements. In our model the U (and V) elements are affected by noise, with a good version of the gray image Y obtainable through existing methods. We show that accurate reconstruction of the chroma components can be accomplished by solving an L1 constrained optimisation problem, where the sparse filter response on natural images is used as a regularization term. This scheme gives comparable results to leading commercial and state of the art denoising algorithms, and exceeds for chroma noise that does not correlate with the luminance structure. Keywords: Natural images, filter response, sparse distributions, denoising, L1 optimisation. 1 INTRODUCTION Some recent algorithms to mention include [LIU08] Denoising is a fundamental problem in image process- where the authors propose a unified framework for two ing due to the fact that images, no matter their content, tasks: automatic estimation and removal of colour noise usually contain some degree of noise. This is often re- from a single image using piecewise smooth image garded as a form of image degradation and the goal of models. -
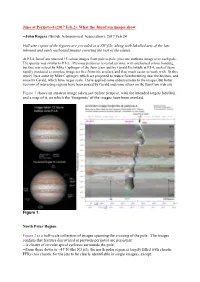
From Jr@Mole
Juno at Perijove-4 (2017 Feb.2): What the JunoCam images show --John Rogers (British Astronomical Association), 2017 Feb.24 Full-size copies of the figures are provided in a ZIP file, along with labelled sets of the late inbound and early outbound images covering the rest of the planet. At PJ-4, JunoCam returned 15 colour images from pole to pole, plus one methane image over each pole. The quality was similar to PJ-3. Previous perijoves revealed an issue with artefactual colour banding, but this was solved by Mike Caplinger of the Juno team and by Gerald Eichstädt; at PJ-4, each of them rapidly produced a complete image set free from this artefact, and thus much easier to work with. In this report, I use some by Mike Caplinger, which are projected to reduce foreshortening near the horizon, and some by Gerald, which have larger scale. I have applied some enhancements to the images, but better versions of interesting regions have been posted by Gerald and some others on the JunoCam web site. Figure 1 shows an amateur image taken just before perijove, with the intended targets labelled, and a map of it, on which the ‘footprints’ of the images have been overlaid. Figure 1. North Polar Region Figure 2 is a half-scale collection of images spanning the crossing of the pole. The images confirm that features discovered at previous perijoves are persistent: --A cluster of circular spiral cyclones surrounds the pole; --From there down to ~43ºN (the N3 jet), the north polar region is largely filled with chaotic FFRs (too chaotic for the jets to be clearly identifiable in single images), except: --A bland zone occupies ~64-68ºN (between the N6 and N7 jets), and has one or more long narrow brown lanes within it, associated with high-level haze near the terminator; --Narrow bands of haze, appearing white near the terminator or across it, are common over all the region.