Prediction of the Amount of Secondary Structure in a Globular Protein from Its Aminoacid Compositionj (Helix/Pl-Sheet/Turns) W
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SUPPLEMENTARY DATA Data Supplement To
SUPPLEMENTARY DATA Data supplement to Perivascular adipose tissue controls insulin-stimulated perfusion, mitochondrial protein expression and glucose uptake in muscle through adipomuscular microvascular anastomoses Surname first author Turaihi Authors & Affiliations Turaihi, Alexander H MD1; Serné, Erik H, MD PhD2; Molthoff, Carla FM PhD3; Koning, Jasper J PhD4; Knol, Jaco PhD6; Niessen, Hans W MD PhD5; Goumans, Marie Jose TH PhD7; van Poelgeest, Erik M MD1; Yudkin, John S MD PhD8; Smulders, Yvo M MD PhD2; Connie R Jimenez, PhD6; van Hinsbergh, Victor WM PhD1; Eringa, Etto C PhD1 ©2020 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-1066/-/DC1 SUPPLEMENTARY DATA Western immunoblotting Skeletal muscle samples were lysed up in 1D‐sample buffer (10% glycerol, 62.5 mmol/L Tris (pH 6.8), 2% w/v LDS, 2% w/v DTT) and protein concentration was determined using Pierce 660‐nm protein assay (Thermo scientific, Waltham, MA USA 02 451; 22 660) according to the manufacturer's instructions. Heat shock protein 90 immunoblotting was performed by application of samples (5 µg protein) on 4‐15% Criterion TGX gels (Biorad, Veenendaal, the Netherlands, 5 671 084) and semi‐dry blotting onto PVDF membranes (GE Healthcare‐Fisher, RPN1416F), incubated overnight with rat monoclonal HSP90 antibody (1:1000 dilution) after blocking with 5% milk in TBS‐T (137 mM NaCl, 20 mmol/L Tris pH 7.0 and 0.1% (v/v) Tween [Sigma‐Aldrich, P7949]). After 2 hours incubation with anti-rat, horse radish peroxidase-coupled secondary antibody (Thermo Fisher 62-9520), the blot was stained using ECL‐prime (Fisher scientific, 10 308 449) and analysed on an AI‐600 imaging system (GE Healthcare, Life Sciences). -
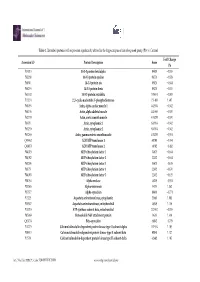
Table 1. Identified Proteins with Expression Significantly Altered in the Hippocampus of Rats of Exposed Group (Pb) Vs
Table 1. Identified proteins with expression significantly altered in the hippocampus of rats of exposed group (Pb) vs. Control. Fold Change Accession Id a Protein Description Score Pb P35213 14-3-3 protein beta/alpha 85420 −0.835 P62260 14-3-3 protein epsilon 96570 −0.878 P68511 14-3-3 protein eta 85420 −0.844 P68255 14-3-3 protein theta 85420 −0.835 P63102 14-3-3 protein zeta/delta 105051 −0.803 P13233 2',3'-cyclic-nucleotide 3'-phosphodiesterase 151400 1.405 P68035 Actin, alpha cardiac muscle 1 442584 −0.942 P68136 Actin, alpha skeletal muscle 441060 −0.970 P62738 Actin, aortic smooth muscle 438270 −0.970 P60711 Actin, cytoplasmic 1 630104 −0.942 P63259 Actin, cytoplasmic 2 630104 −0.942 P63269 Actin, gamma-enteric smooth muscle 438270 −0.951 Q05962 ADP/ATP translocase 1 60100 −0.554 Q09073 ADP/ATP translocase 2 49102 −0.482 P84079 ADP-ribosylation factor 1 34675 −0.644 P84082 ADP-ribosylation factor 2 22412 −0.644 P61206 ADP-ribosylation factor 3 34675 −0.619 P61751 ADP-ribosylation factor 4 22412 −0.670 P84083 ADP-ribosylation factor 5 22412 −0.625 P04764 Alpha-enolase 46219 −0.951 P23565 Alpha-internexin 9478 1.062 P37377 Alpha-synuclein 89619 −0.771 P13221 Aspartate aminotransferase, cytoplasmic 23661 1.083 P00507 Aspartate aminotransferase, mitochondrial 46049 1.116 P10719 ATP synthase subunit beta, mitochondrial 232442 −0.835 P85969 Beta-soluble NSF attachment protein 9638 1.419 Q63754 Beta-synuclein 66842 −0.779 P11275 Calcium/calmodulin-dependent protein kinase type II subunit alpha 181954 1.105 P08413 Calcium/calmodulin-dependent protein kinase type II subunit beta 80840 1.127 P15791 Calcium/calmodulin-dependent protein kinase type II subunit delta 62682 1.105 Int. -

Families and the Structural Relatedness Among Globular Proteins
Protein Science (1993), 2, 884-899. Cambridge University Press. Printed in the USA. Copyright 0 1993 The Protein Society -~~ ~~~~ ~ Families and the structural relatedness among globular proteins DAVID P. YEE AND KEN A. DILL Department of Pharmaceutical Chemistry, University of California, San Francisco, California94143-1204 (RECEIVEDJanuary 6, 1993; REVISEDMANUSCRIPT RECEIVED February 18, 1993) Abstract Protein structures come in families. Are families “closely knit” or “loosely knit” entities? We describe a mea- sure of relatedness among polymer conformations. Based on weighted distance maps, this measure differs from existing measures mainly in two respects: (1) it is computationally fast, and (2) it can compare any two proteins, regardless of their relative chain lengths or degree of similarity. It does not require finding relative alignments. The measure is used here to determine the dissimilarities between all 12,403 possible pairs of 158 diverse protein structures from the Brookhaven Protein Data Bank (PDB). Combined with minimal spanning trees and hier- archical clustering methods,this measure is used to define structural families. It is also useful for rapidly searching a dataset of protein structures for specific substructural motifs.By using an analogy to distributions of Euclid- ean distances, we find that protein families are not tightly knit entities. Keywords: protein family; relatedness; structural comparison; substructure searches Pioneering work over the past 20 years has shown that positions after superposition. RMS is a useful distance proteins fall into families of related structures (Levitt & metric for comparingstructures that arenearly identical: Chothia, 1976; Richardson, 1981; Richardson & Richard- for example, when refining or comparing structures ob- son, 1989; Chothia & Finkelstein, 1990). -

Iron Deficiency in the Rat: Effects on Neutrophil Activation and Metabolism
THEOPHYLLINE AND BRAIN 549 A dietary protein deficiency can affect the susceptibility to the metabolism in children with protein-calorie malnutrition. Am. J. Clin. Nutr., 28: 977 (1975). toxicity of drugs or other agents such as pesticides and herbicides 8. Nakamoto. T. and Miller. S. A.: Effect of vrotein-energy malnutrition on the (2). At the present time, there are no such studies relative to growth df mandible aid long bone in newborn miie and female rats. J. theophylline, although the usage of theophylline is now quite Nutr., 107: 983 (1977). common in the neonatal intensive care environment. Many 9. Nebron, R. M., Resnick, M. D., and Halstmm, W. J.: Developmental outcome of premature infants treated with theophylline. Dev. Pharmacol. Ther., 1: infants therein who are now receiving theophylline therapeuti- 274 ( 1980). cally are being dosed on a body weight basis without considera- 10. Newberne, P. M., Gross, R. L., and Roe, D. A,: Dmg, toxin, nutrient interac- tion of the nutritional status. Our data suggest that, in the animal tions. World Rev. Nutr. Diet., 29: 130 (1978). model, the administration of theophylline in the presence of a 1 I. Pastorova, B., Sova, O., and Burda, J.: Incorporation of I4C-thymidine into liver and brain DNA of protein-deficient rats. Physiol. Bohemoslov., 27: 69 compromised nutritional status may have effects not now appar- (1978). ent. We add our concern to that expressed by others (16) that 12. Prasad, A. S., Dumouchell, E., Kovich, D., and Oberleas, D.: A simple methylxanthine administration may have previously unsus- fluorometric method for the determination of RNA and DNA in tissue. -

The Effects of Temperature on Hemoglobin in Capitella Teleta
THE EFFECTS OF TEMPERATURE ON HEMOGLOBIN IN CAPITELLA TELETA by Alexander M. Barclay A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for the degree of Master of Science in Marine Studies Summer 2013 c 2013 Alexander M. Barclay All Rights Reserved THE EFFECTS OF TEMPERATURE ON HEMOGLOBIN IN CAPITELLA TELETA by Alexander M. Barclay Approved: Adam G. Marsh, Ph.D. Professor in charge of thesis on behalf of the Advisory Committee Approved: Mark A. Moline, Ph.D. Director of the School of Marine Science and Policy Approved: Nancy M. Targett, Ph.D. Dean of the College of Earth, Ocean, and Environment Approved: James G. Richards, Ph.D. Vice Provost for Graduate and Professional Education ACKNOWLEDGMENTS I extend my sincere gratitude to the individuals that either contributed to the execution of my thesis project or to my experience here at the University of Delaware. I would like to give special thanks to my adviser, Adam Marsh, who afforded me an opportunity that exceeded all of my prior expectations. Adam will say that I worked very independently and did not require much guidance, but he served as an inspiration and a role model for me during my studies. Adam sincerely cares for each of his students and takes the time and thought to tailor his research program to fit each person's interests. The reason that I initially chose to work in Adam's lab was that he expressed a genuine excitement for science and for his lifes work. His enthusiasm resonated with me and helped me to find my own exciting path in science. -

Chain of Human Neutrophil Cytochrome B CHARLES A
Proc. Nati. Acad. Sci. USA Vol. 85, pp. 3319-3323, May 1988 Biochemistry Primary structure and unique expression of the 22-kilodalton light chain of human neutrophil cytochrome b CHARLES A. PARKOS*, MARY C. DINAUERt, LESLIE E. WALKER*, RODGER A. ALLEN*, ALGIRDAS J. JESAITIS*, AND STUART H. ORKINtt *Department of Immunology, Research Institute of the Scripps Clinic, La Jolla, CA 92037; tDivision of Hematology-Oncology, Children's Hospital, and Dana-Farber Cancer Institute, Department of Pediatrics, Harvard Medical School, Boston, MA 02115; and tHoward Hughes Medical Institute, Children's Hospital, Boston, MA 02115 Communicated by Harvey F. Lodish, January 14, 1988 ABSTRACT Cytochrome b comprising 91-kDa and 22- Cytochrome b purified from neutrophil membranes ap- kDa subunits is a critical component of the membrane-bound pears to be a heterodimer of a glycosylated 91-kDa heavy oxidase of phagocytes that generates superoxide. This impor- chain and a nonglycosylated 22-kDa light chain (10-12). The tant microbicidal system is impaired in inherited disorders 91-kDa subunit is encoded by a gene designated CGD, known as chronic granulomatous disease (CGD). Previously we residing at chromosomal position Xp2l, which originally was determined the sequence of the larger subunit from the cDNA identified on the basis of genetic linkage without reference to of the CGD gene, the X chromosome locus affected in "X- a specific protein product (8). Antisera generated to either a linked" CGD. To complete the primary structure of the synthetic peptide predicted from the cDNA or to a fusion cytochrome b and to assess expression of the smaller subunit, protein produced in E. -

Table S1. Identified Proteins with Exclusive Expression in Cerebellum of Rats of Control, 10Mg F/L and 50Mg F/L Groups
Table S1. Identified proteins with exclusive expression in cerebellum of rats of control, 10mg F/L and 50mg F/L groups. Accession PLGS Protein Name Group IDa Score Q3TXS7 26S proteasome non-ATPase regulatory subunit 1 435 Control Q9CQX8 28S ribosomal protein S36_ mitochondrial 197 Control P52760 2-iminobutanoate/2-iminopropanoate deaminase 315 Control Q60597 2-oxoglutarate dehydrogenase_ mitochondrial 67 Control P24815 3 beta-hydroxysteroid dehydrogenase/Delta 5-->4-isomerase type 1 84 Control Q99L13 3-hydroxyisobutyrate dehydrogenase_ mitochondrial 114 Control P61922 4-aminobutyrate aminotransferase_ mitochondrial 470 Control P10852 4F2 cell-surface antigen heavy chain 220 Control Q8K010 5-oxoprolinase 197 Control P47955 60S acidic ribosomal protein P1 190 Control P70266 6-phosphofructo-2-kinase/fructose-2_6-bisphosphatase 1 113 Control Q8QZT1 Acetyl-CoA acetyltransferase_ mitochondrial 402 Control Q9R0Y5 Adenylate kinase isoenzyme 1 623 Control Q80TS3 Adhesion G protein-coupled receptor L3 59 Control B7ZCC9 Adhesion G-protein coupled receptor G4 139 Control Q6P5E6 ADP-ribosylation factor-binding protein GGA2 45 Control E9Q394 A-kinase anchor protein 13 60 Control Q80Y20 Alkylated DNA repair protein alkB homolog 8 111 Control P07758 Alpha-1-antitrypsin 1-1 78 Control P22599 Alpha-1-antitrypsin 1-2 78 Control Q00896 Alpha-1-antitrypsin 1-3 78 Control Q00897 Alpha-1-antitrypsin 1-4 78 Control P57780 Alpha-actinin-4 58 Control Q9QYC0 Alpha-adducin 270 Control Q9DB05 Alpha-soluble NSF attachment protein 156 Control Q6PAM1 Alpha-taxilin 161 -

Myoglobin-Mediated Oxygen Delivery to Mitochondria of Isolated Cardiac Myocytes (Electron Transport/Heart Cells/Cytochrome Oxidase) BEATRICE A
Proc. Nati. Acad. Sci. USA Vol. 84, pp. 7503-7507, November 1987 Biochemistry Myoglobin-mediated oxygen delivery to mitochondria of isolated cardiac myocytes (electron transport/heart cells/cytochrome oxidase) BEATRICE A. WITTENBERG* AND JONATHAN B. WITTENBERG Department of Physiology and Biophysics, Albert Einstein College of Medicine, Bronx, NY 10461 Communicated by Berta Scharrer, July 20, 1987 (receivedfor review May 5, 1987) ABSTRACT Myoglobin-mediated oxygen delivery to in- Cytochrome oxidase, half-oxidized when ambient oxygen tracellular mitochondria is demonstrated in cardiac myocytes partial pressure (Po2) is 0.07 torr (1 torr = 133 Pa) (16), in the isolated from the hearts of mature rats. Myocytes are held at circumstance described here experiences oxygen pressures high ambient oxygen pressure, 40-340 torr (5-45 kPa); 20- to 200-fold the pressure required to maintain the normal, sarcoplasmic myoglobin is fully oxygenated. In this condition largely oxidized, state seen in resting myocytes (16). Carbon oxygen availability does not limit respiratory rate; myoglobin- monoxide in this circumstance blocks oxygenation of facilitated diffusion contributes no additional oxygen flux and, sarcoplasmic myoglobin selectively without perturbing the since oxygen consumption is measured in steady states, the optical spectrum of intracellular cytochrome oxidase. We storage function of myoglobin vanishes. Carbon monoxide, conclude that cardiac mitochondria accept two additive introduced stepwise, displaces oxygen from intracellular simultaneous flows of oxygen: the well-known flow of dis- oxymyoglobin without altering the optical spectrum of the solved oxygen to cytochrome oxidase and a flow of largely oxidized intracellular mitochondria. A large part, myoglobin-bound oxygen to a mitochondrial terminus. The about one-third, of the steady-state oxygen uptake is abolished myoglobin-mediated oxygen flow supports ATP generation by carbon monoxide blockade of myoglobin oxygenation. -

Liver Mitochondrial DNA Damage and Genetic Variability of Cytochrome B - a Key Component of the Respirasome - Drive the Severity of Fatty Liver Disease
DR. SILVIA SOOKOIAN (Orcid ID : 0000-0001-5929-5470) Article type : Original This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differencesAccepted Article between this version and the Version of Record. Please cite this article as doi: 10.1111/JOIM.13147 This article is protected by copyright. All rights reserved Liver mitochondrial DNA damage and genetic variability of Cytochrome b - a key component of the respirasome - drive the severity of fatty liver disease Short title: NASH and respirasome Carlos J Pirola *, Martin Garaycoechea, Diego Flichman, Gustavo O Castaño and Silvia Sookoian *. 1 University of Buenos Aires, School of Medicine, Institute of Medical Research A Lanari, Ciudad Autónoma de Buenos Aires, Argentina (CJP, SS). 2 National Scientific and Technical Research Council (CONICET)−University of Buenos Aires, Institute of Medical Research (IDIM), Department of Molecular Genetics and Biology of Complex Diseases, Ciudad Autónoma de Buenos Aires, Argentina (CJP). 3 Department of Surgery, Hospital de Alta Complejidad en Red “El Cruce”, Florencio Varela, Buenos Aires, Argentina (MG). 4 Department of Virology, School of Pharmacy and Biochemistry, University of Buenos Aires, Ciudad Autónoma de Buenos Aires, Argentina (DF). 5 Liver Unit, Medicine and Surgery Department, Hospital Abel Zubizarreta, Ciudad Autónoma de Buenos Aires, Argentina (GOC). 6 National Scientific and Technical Research Council (CONICET)−University of Buenos Aires, Institute of Medical Research (IDIM), Department of Clinical and Molecular Hepatology, Ciudad Autónoma de Buenos Aires, Argentina (SS). * Co-corresponding authorship Grant support: This study was partially supported by grants PICT 2015-0551 and PICT 2016- 0135 (Agencia Nacional de Promoción Científica y Tecnológica, FONCyT). -

Molecular Evolution of Cytochrome B in High- and Low-Altitude Deer Mice (Genus Peromyscus)
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Jay F. Storz Publications Papers in the Biological Sciences 2008 Molecular evolution of cytochrome b in high- and low-altitude deer mice (genus Peromyscus) E.J. Gering University of Texas, 1 University Station C0930, Austin, TX, [email protected] J. C. Opazo Instituto de Ecologia y Evolucion, Facultad de Ciencias, Universidad Austral de Chile, Casilla 567, Valdivia, Chile Jay F. Storz University of Nebraska - Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/bioscistorz Part of the Genetics and Genomics Commons Gering, E.J.; Opazo, J. C.; and Storz, Jay F., "Molecular evolution of cytochrome b in high- and low-altitude deer mice (genus Peromyscus)" (2008). Jay F. Storz Publications. 23. https://digitalcommons.unl.edu/bioscistorz/23 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Jay F. Storz Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Published in Heredity (2008), doi: 10.1038/hdy.2008.124 Copyright © 2008 The Genetics Society; published by Macmillan/Nature Publishing Group. Used by permision. http://www.nature.com/hdy/index.html Submitted March 19, 2008; revised November 11, 2008; accepted November 21, 2008; published online December 24, 2008. Molecular evolution of cytochrome b in high- and low-altitude deer mice (genus Peromyscus) E. J. Gering,* J. C. Opazo,† and J. F. Storz School of Biological Sciences, University of Nebraska–Lincoln, Lincoln, NE, USA * Corresponding author — current address: Section of Integrative Biology, University of Texas, 1 University Station C0930, Austin, TX 78712, USA; email [email protected] † Current address: Instituto de Ecologia y Evolucion, Facultad de Ciencias, Universidad Austral de Chile, Casilla 567, Valdivia, Chile. -

Molecular and Whole Animal Responses of Grass Shrimp, Palaemonetes Pugio, Exposed to Chronic Hypoxia ⁎ Marius Brouwer A, , Nancy J
Journal of Experimental Marine Biology and Ecology 341 (2007) 16–31 www.elsevier.com/locate/jembe Molecular and whole animal responses of grass shrimp, Palaemonetes pugio, exposed to chronic hypoxia ⁎ Marius Brouwer a, , Nancy J. Brown-Peterson a, Patrick Larkin b, Vishal Patel c, Nancy Denslow c, Steve Manning a, Theodora Hoexum Brouwer a a Department of Coastal Sciences, The University of Southern Mississippi, 703 East Beach Dr., Ocean Springs, MS 39564, USA b EcoArray Inc., 12085 Research Dr., Alachua, Florida 32615, USA c Department of Physiological Sciences and Center for Environmental and Human Toxicology, University of Florida, PO Box 110885, Gainesville, FL 32611, USA Received 28 July 2006; received in revised form 15 September 2006; accepted 20 October 2006 Abstract Hypoxic conditions in estuaries are one of the major factors responsible for the declines in habitat quality. Previous studies examining effects of hypoxia on crustacea have focused on individual/population-level, physiological or molecular responses but have not considered more than one type of response in the same study. The objective of this study was to examine responses of grass shrimp, Palaemonetes pugio, to moderate (2.5 ppm DO) and severe (1.5 ppm DO) chronic hypoxia at both the molecular and organismal levels. At the molecular level we measured hypoxia-induced alterations in gene expression using custom cDNA macroarrays containing 78 clones from a hypoxia- responsive suppression subtractive hybridization cDNA library. Grass shrimp exposed to moderate hypoxia show minimal changes in gene expression. The response after short-term (3 d) exposure to severe hypoxia was up-regulation of genes involved in oxygen uptake/transport and energy production, such as hemocyanin and ATP synthases. -

82182065.Pdf
A42 HEMOGLOBINHIEMOGLOBIN AND MYOGLODINISYOGLOBIN M-Poe107 MPos108 LOW TEMPERATURE RECOMBINATION OF MbO, USING FOURIER TIME-RESOLVED X-RAY ABSORPTION SPECTROSCOPY ON A 5 ps TRANSFORM INFRARED SPECTROSCOPY. ((M. Halterman, L. M. Miller, and TIMESCALE. ((M.R. Chance, M.D. Wort, L.M. Miller, E.M. Scheuring, A. Xie, M. R. Chance)) Albert Einstein College of Medicine., Bronx, NY 10461. D.E. Sidelinger)) Albert Einstein Col of Med., Bronx, NY 10461 The binding of oxygen to hemoglobin and myoglobin is one of the most X-ray absorption spectroscopy (XAS) is unmatched in measuring metal-ligand important reactions in human cells. Essential to understanding the binding distances in solution while the dynamics of evolving systems is a subject of process is the study of intermediate states. To date, the intermediate states intense interest in biophysics currently. We report the design and successful involved in carbon monoxide binding are much better understood than those testing of a system for collecting time-resolved XAS data on a 5ps timescale. of oxygen binding. However, recent studies have shown that 02 and CO have The system is based on the use of a germanium energy resolving detector and significantly different rebinding intermediates (M. Chance, et. al., Biochemistry, a fast multichannel scaling (FMS) board, both from Canberra Ind. The output 29, 1990, p. 5537). Vibrational spectroscopic methods have indicated several of a single Ge channel is amplified and connected to the FMS board. The data bands for MbO2 ligand stretching modes (Potter, et. al., Biochemistry, 26, acquisition sequence is controlled by a digital delay generator, which also 1987, p.