Duodenal Cytochrome B (DCYTB) in Iron Metabolism: an Update on Function and Regulation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SUPPLEMENTARY DATA Data Supplement To
SUPPLEMENTARY DATA Data supplement to Perivascular adipose tissue controls insulin-stimulated perfusion, mitochondrial protein expression and glucose uptake in muscle through adipomuscular microvascular anastomoses Surname first author Turaihi Authors & Affiliations Turaihi, Alexander H MD1; Serné, Erik H, MD PhD2; Molthoff, Carla FM PhD3; Koning, Jasper J PhD4; Knol, Jaco PhD6; Niessen, Hans W MD PhD5; Goumans, Marie Jose TH PhD7; van Poelgeest, Erik M MD1; Yudkin, John S MD PhD8; Smulders, Yvo M MD PhD2; Connie R Jimenez, PhD6; van Hinsbergh, Victor WM PhD1; Eringa, Etto C PhD1 ©2020 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-1066/-/DC1 SUPPLEMENTARY DATA Western immunoblotting Skeletal muscle samples were lysed up in 1D‐sample buffer (10% glycerol, 62.5 mmol/L Tris (pH 6.8), 2% w/v LDS, 2% w/v DTT) and protein concentration was determined using Pierce 660‐nm protein assay (Thermo scientific, Waltham, MA USA 02 451; 22 660) according to the manufacturer's instructions. Heat shock protein 90 immunoblotting was performed by application of samples (5 µg protein) on 4‐15% Criterion TGX gels (Biorad, Veenendaal, the Netherlands, 5 671 084) and semi‐dry blotting onto PVDF membranes (GE Healthcare‐Fisher, RPN1416F), incubated overnight with rat monoclonal HSP90 antibody (1:1000 dilution) after blocking with 5% milk in TBS‐T (137 mM NaCl, 20 mmol/L Tris pH 7.0 and 0.1% (v/v) Tween [Sigma‐Aldrich, P7949]). After 2 hours incubation with anti-rat, horse radish peroxidase-coupled secondary antibody (Thermo Fisher 62-9520), the blot was stained using ECL‐prime (Fisher scientific, 10 308 449) and analysed on an AI‐600 imaging system (GE Healthcare, Life Sciences). -
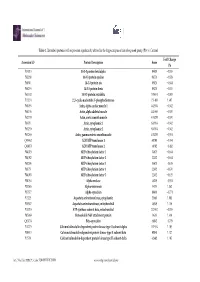
Table 1. Identified Proteins with Expression Significantly Altered in the Hippocampus of Rats of Exposed Group (Pb) Vs
Table 1. Identified proteins with expression significantly altered in the hippocampus of rats of exposed group (Pb) vs. Control. Fold Change Accession Id a Protein Description Score Pb P35213 14-3-3 protein beta/alpha 85420 −0.835 P62260 14-3-3 protein epsilon 96570 −0.878 P68511 14-3-3 protein eta 85420 −0.844 P68255 14-3-3 protein theta 85420 −0.835 P63102 14-3-3 protein zeta/delta 105051 −0.803 P13233 2',3'-cyclic-nucleotide 3'-phosphodiesterase 151400 1.405 P68035 Actin, alpha cardiac muscle 1 442584 −0.942 P68136 Actin, alpha skeletal muscle 441060 −0.970 P62738 Actin, aortic smooth muscle 438270 −0.970 P60711 Actin, cytoplasmic 1 630104 −0.942 P63259 Actin, cytoplasmic 2 630104 −0.942 P63269 Actin, gamma-enteric smooth muscle 438270 −0.951 Q05962 ADP/ATP translocase 1 60100 −0.554 Q09073 ADP/ATP translocase 2 49102 −0.482 P84079 ADP-ribosylation factor 1 34675 −0.644 P84082 ADP-ribosylation factor 2 22412 −0.644 P61206 ADP-ribosylation factor 3 34675 −0.619 P61751 ADP-ribosylation factor 4 22412 −0.670 P84083 ADP-ribosylation factor 5 22412 −0.625 P04764 Alpha-enolase 46219 −0.951 P23565 Alpha-internexin 9478 1.062 P37377 Alpha-synuclein 89619 −0.771 P13221 Aspartate aminotransferase, cytoplasmic 23661 1.083 P00507 Aspartate aminotransferase, mitochondrial 46049 1.116 P10719 ATP synthase subunit beta, mitochondrial 232442 −0.835 P85969 Beta-soluble NSF attachment protein 9638 1.419 Q63754 Beta-synuclein 66842 −0.779 P11275 Calcium/calmodulin-dependent protein kinase type II subunit alpha 181954 1.105 P08413 Calcium/calmodulin-dependent protein kinase type II subunit beta 80840 1.127 P15791 Calcium/calmodulin-dependent protein kinase type II subunit delta 62682 1.105 Int. -

Iron Deficiency in the Rat: Effects on Neutrophil Activation and Metabolism
THEOPHYLLINE AND BRAIN 549 A dietary protein deficiency can affect the susceptibility to the metabolism in children with protein-calorie malnutrition. Am. J. Clin. Nutr., 28: 977 (1975). toxicity of drugs or other agents such as pesticides and herbicides 8. Nakamoto. T. and Miller. S. A.: Effect of vrotein-energy malnutrition on the (2). At the present time, there are no such studies relative to growth df mandible aid long bone in newborn miie and female rats. J. theophylline, although the usage of theophylline is now quite Nutr., 107: 983 (1977). common in the neonatal intensive care environment. Many 9. Nebron, R. M., Resnick, M. D., and Halstmm, W. J.: Developmental outcome of premature infants treated with theophylline. Dev. Pharmacol. Ther., 1: infants therein who are now receiving theophylline therapeuti- 274 ( 1980). cally are being dosed on a body weight basis without considera- 10. Newberne, P. M., Gross, R. L., and Roe, D. A,: Dmg, toxin, nutrient interac- tion of the nutritional status. Our data suggest that, in the animal tions. World Rev. Nutr. Diet., 29: 130 (1978). model, the administration of theophylline in the presence of a 1 I. Pastorova, B., Sova, O., and Burda, J.: Incorporation of I4C-thymidine into liver and brain DNA of protein-deficient rats. Physiol. Bohemoslov., 27: 69 compromised nutritional status may have effects not now appar- (1978). ent. We add our concern to that expressed by others (16) that 12. Prasad, A. S., Dumouchell, E., Kovich, D., and Oberleas, D.: A simple methylxanthine administration may have previously unsus- fluorometric method for the determination of RNA and DNA in tissue. -

Curriculum Vitae
CURRICULUM VITAE PART I: General Information DATE PREPARED: 1/2018 Name: KARL TIMOTHY KELSEY 70 Ship Street, G-E3, Department of Epidemiology and Pathology and Lab. Office Address: Medicine, Providence, RI 02912 Home Address: 57 Toxteth Street, Brookline, MA 02446 Work E-mail: [email protected] Work FAX: (401) 863-5132 Place of Birth: Minneapolis, Minnesota Education: 1976 B.A., Physics University of Minnesota, Minneapolis, MN 1981 M.D., Medicine University of Minnesota, Minneapolis, MN 1984 M.O.H, Occupational Health Harvard University, Boston, MA Postdoctoral Training: 1981–1982 Resident Diagnostic Radiology Mt. Zion Hospital and Medical Center, San Francisco, CA 1982–1983 Postdoctoral Fellow Environmental Pathology Laboratory of Environmental Pathology University of Minnesota Medical School Minneapolis, Minnesota 1983–1985 Resident Occupational Medicine Harvard School of Public Health, Boston, MA 1985–1987 Postdoctoral Fellow Environmental Carcinogenesis Laboratory of Radiobiology Harvard School of Public Health Boston, Massachusetts Licensure and Certification: 1982 Minnesota Medical License (inactive by choice) 1984 Massachusetts Medical License (inactive by choice) 1986 Board Certification, American Board of Preventive Medicine – Occupational Medicine Academic Appointments: 1986–1987 Lecturer of Community Medicine Tufts University School of Medicine 1987–1991 Assistant Professor of Occupational Medicine Harvard School of Public Health 1988–1991 Assistant Professor of Radiobiology Harvard School of Public Health 1991–1998 Associate Professor of Occupational Medicine Harvard School of Public Health 1991–1998 Associate Professor of Radiobiology Harvard School of Public Health 1995–2007 Associate Physician of Channing Laboratory Brigham and Women’s Hospital 1995–2007 Associate Professor of Medicine Harvard Medical School 1998–2007 Professor of Cancer Biology & Environmental Health Harvard School of Public Health 2007-pres. -

Duodenal Mrna Expression of Iron Related Genes in Response To
648 SMALL INTESTINE Duodenal mRNA expression of iron related genes in Gut: first published as 10.1136/gut.51.5.648 on 1 November 2002. Downloaded from response to iron loading and iron deficiency in four strains of mice F Dupic, S Fruchon, M Bensaid, O Loreal, P Brissot, N Borot, M P Roth, H Coppin ............................................................................................................................. Gut 2002;51:648–653 Background: Although much progress has been made recently in characterising the proteins involved in duodenal iron trafficking, regulation of intestinal iron transport remains poorly understood. It is not known whether the level of mRNA expression of these recently described molecules is genetically regu- lated. This is of particular interest however as genetic factors are likely to determine differences in iron status among mouse strains and probably also contribute to the phenotypic variability seen with disrup- tion of the haemochromatosis gene. Aims: To investigate this issue, we examined concomitant variations in duodenal cytochrome b (Dcytb), divalent metal transporter 1 (DMT1), ferroportin 1 (FPN1), hephaestin, stimulator of Fe trans- port (SFT), HFE, and transferrin receptor 1 (TfR1) transcripts in response to different dietary iron contents in the four mouse strains C57BL/6, DBA/2, CBA, and 129/Sv. See end of article for authors’ affiliations Subjects: Six mice of each strain were fed normal levels of dietary iron, six were subjected to the same ....................... diet supplemented with -

Chain of Human Neutrophil Cytochrome B CHARLES A
Proc. Nati. Acad. Sci. USA Vol. 85, pp. 3319-3323, May 1988 Biochemistry Primary structure and unique expression of the 22-kilodalton light chain of human neutrophil cytochrome b CHARLES A. PARKOS*, MARY C. DINAUERt, LESLIE E. WALKER*, RODGER A. ALLEN*, ALGIRDAS J. JESAITIS*, AND STUART H. ORKINtt *Department of Immunology, Research Institute of the Scripps Clinic, La Jolla, CA 92037; tDivision of Hematology-Oncology, Children's Hospital, and Dana-Farber Cancer Institute, Department of Pediatrics, Harvard Medical School, Boston, MA 02115; and tHoward Hughes Medical Institute, Children's Hospital, Boston, MA 02115 Communicated by Harvey F. Lodish, January 14, 1988 ABSTRACT Cytochrome b comprising 91-kDa and 22- Cytochrome b purified from neutrophil membranes ap- kDa subunits is a critical component of the membrane-bound pears to be a heterodimer of a glycosylated 91-kDa heavy oxidase of phagocytes that generates superoxide. This impor- chain and a nonglycosylated 22-kDa light chain (10-12). The tant microbicidal system is impaired in inherited disorders 91-kDa subunit is encoded by a gene designated CGD, known as chronic granulomatous disease (CGD). Previously we residing at chromosomal position Xp2l, which originally was determined the sequence of the larger subunit from the cDNA identified on the basis of genetic linkage without reference to of the CGD gene, the X chromosome locus affected in "X- a specific protein product (8). Antisera generated to either a linked" CGD. To complete the primary structure of the synthetic peptide predicted from the cDNA or to a fusion cytochrome b and to assess expression of the smaller subunit, protein produced in E. -

Table S1. Identified Proteins with Exclusive Expression in Cerebellum of Rats of Control, 10Mg F/L and 50Mg F/L Groups
Table S1. Identified proteins with exclusive expression in cerebellum of rats of control, 10mg F/L and 50mg F/L groups. Accession PLGS Protein Name Group IDa Score Q3TXS7 26S proteasome non-ATPase regulatory subunit 1 435 Control Q9CQX8 28S ribosomal protein S36_ mitochondrial 197 Control P52760 2-iminobutanoate/2-iminopropanoate deaminase 315 Control Q60597 2-oxoglutarate dehydrogenase_ mitochondrial 67 Control P24815 3 beta-hydroxysteroid dehydrogenase/Delta 5-->4-isomerase type 1 84 Control Q99L13 3-hydroxyisobutyrate dehydrogenase_ mitochondrial 114 Control P61922 4-aminobutyrate aminotransferase_ mitochondrial 470 Control P10852 4F2 cell-surface antigen heavy chain 220 Control Q8K010 5-oxoprolinase 197 Control P47955 60S acidic ribosomal protein P1 190 Control P70266 6-phosphofructo-2-kinase/fructose-2_6-bisphosphatase 1 113 Control Q8QZT1 Acetyl-CoA acetyltransferase_ mitochondrial 402 Control Q9R0Y5 Adenylate kinase isoenzyme 1 623 Control Q80TS3 Adhesion G protein-coupled receptor L3 59 Control B7ZCC9 Adhesion G-protein coupled receptor G4 139 Control Q6P5E6 ADP-ribosylation factor-binding protein GGA2 45 Control E9Q394 A-kinase anchor protein 13 60 Control Q80Y20 Alkylated DNA repair protein alkB homolog 8 111 Control P07758 Alpha-1-antitrypsin 1-1 78 Control P22599 Alpha-1-antitrypsin 1-2 78 Control Q00896 Alpha-1-antitrypsin 1-3 78 Control Q00897 Alpha-1-antitrypsin 1-4 78 Control P57780 Alpha-actinin-4 58 Control Q9QYC0 Alpha-adducin 270 Control Q9DB05 Alpha-soluble NSF attachment protein 156 Control Q6PAM1 Alpha-taxilin 161 -

Publications 2002 Agarwal, R., Peters, T.J., Coombes, R.C., Vigushin, D.M
Publications 2002 Agarwal, R., Peters, T.J., Coombes, R.C., Vigushin, D.M. Tamoaxifen-related porphyria cutanea tarda. Medical Oncology. 19, 121-124 (2002) Anderson GJ, Frazer DM, McKie AT, Vulpe CD. The ceruloplasmin homolog hephaestin and the control of intestinal iron absorption. Blood Cells Mol Dis. 2002 Nov-Dec;29(3):367-75. Anderson GJ, Frazer DM, Wilkins SJ, Becker EM, Millard KN, Murphy TL, McKie AT, Vulpe CD. Relationship between intestinal iron-transporter expression, hepatic hepcidin levels and the control of iron absorption. Biochem Soc Trans. 2002 Aug;30(4):724-6. Anderson GJ, Frazer DM, McKie AT, Wilkins SJ, Vulpe CD. The expression and regulation of the iron transport molecules hephaestin and IREG1: implications for the control of iron export from the small intestine. Cell Biochem Biophys. 2002;36(2- 3):137-46. Review. Cremonesi, P., Acebron, A., Raja, K.B., & Simpson, R.J. Iron absorption: Biochemical and molecular insights into the importance of iron species for intestinal uptake. Pharmacol Toxicol, 91, 97-102 (2002) Desouky M, Jugdaohsingh R, McCrohan CR, White KN, Powell JJ. Aluminium- Dependent Regulation of Intracellular Silicon in the Aquatic Invertebrate Lymnaea stagnalis Proc. Natl. Acad. Sci. 2002;99:3394-3399. Evans RW, Hider RC & Wrigglesworth JM. (2002). Biometals 2002: Third International Biometals Symposium, Biochemical Society Transactions 30, 669-783. Evans, R.W.& Oakhill, J.S. (2002) Transferrin-mediated iron acquisition by pathogenic Neisseria. Biochemical Society, 30, 705-707. Frazer DM, Wilkins SJ, Becker EM, Vulpe CD, McKie AT, Trinder D, Anderson GJ. Hepcidin expression inversely correlates with the expression of duodenal iron transporters and iron absorption in rats. -

Seed Storage Proteins
SEED STORAGE PROTEINS PRESENTED BY: DIVYA KAUSHIK M.Sc BIOTECHNOLOGY 2nd SEMESTER INTRODUCTION The plant seed is not only an organ of propagation and dispersal but also the major plant tissue harvested by humankind. The amount of protein present in seeds varies from 10% (in cereals) to 40% (in certain legumes and oilseeds) of the dry weight, forming a major source of dietary protein. Although the vast majority of the individual proteins present in mature seeds have either metabolic or structural roles, all seeds also contain one or more groups of proteins that are present in high amounts and that serve to provide a store of amino acids for use during germination and seedling growth. These storage proteins are of particular importance because they determine not only the total protein content of the seed but also its quality for various end uses. CHARACTERISTICS OF SEED STORAGE PROTEINS Seed storage proteins (SSPs) are specifically synthesized during seed maturation and accumulate in the endosperm of monocots or in the cotyledons and embryos of dicots. Their presence in mature seeds in discrete deposit are called protein bodies. One of the earliest and first isolated proteins is wheat gluten and Brazil nut globulin. They are synthesized at high levels in specific tissues and at certain stages of development. their synthesis is regulated by nutrition, and they act as a sink for surplus nitrogen. CLASSIFICATION Classification of proteins in to their groups is based on their solubility. Three protein groups have been categorized during -

Myoglobin-Mediated Oxygen Delivery to Mitochondria of Isolated Cardiac Myocytes (Electron Transport/Heart Cells/Cytochrome Oxidase) BEATRICE A
Proc. Nati. Acad. Sci. USA Vol. 84, pp. 7503-7507, November 1987 Biochemistry Myoglobin-mediated oxygen delivery to mitochondria of isolated cardiac myocytes (electron transport/heart cells/cytochrome oxidase) BEATRICE A. WITTENBERG* AND JONATHAN B. WITTENBERG Department of Physiology and Biophysics, Albert Einstein College of Medicine, Bronx, NY 10461 Communicated by Berta Scharrer, July 20, 1987 (receivedfor review May 5, 1987) ABSTRACT Myoglobin-mediated oxygen delivery to in- Cytochrome oxidase, half-oxidized when ambient oxygen tracellular mitochondria is demonstrated in cardiac myocytes partial pressure (Po2) is 0.07 torr (1 torr = 133 Pa) (16), in the isolated from the hearts of mature rats. Myocytes are held at circumstance described here experiences oxygen pressures high ambient oxygen pressure, 40-340 torr (5-45 kPa); 20- to 200-fold the pressure required to maintain the normal, sarcoplasmic myoglobin is fully oxygenated. In this condition largely oxidized, state seen in resting myocytes (16). Carbon oxygen availability does not limit respiratory rate; myoglobin- monoxide in this circumstance blocks oxygenation of facilitated diffusion contributes no additional oxygen flux and, sarcoplasmic myoglobin selectively without perturbing the since oxygen consumption is measured in steady states, the optical spectrum of intracellular cytochrome oxidase. We storage function of myoglobin vanishes. Carbon monoxide, conclude that cardiac mitochondria accept two additive introduced stepwise, displaces oxygen from intracellular simultaneous flows of oxygen: the well-known flow of dis- oxymyoglobin without altering the optical spectrum of the solved oxygen to cytochrome oxidase and a flow of largely oxidized intracellular mitochondria. A large part, myoglobin-bound oxygen to a mitochondrial terminus. The about one-third, of the steady-state oxygen uptake is abolished myoglobin-mediated oxygen flow supports ATP generation by carbon monoxide blockade of myoglobin oxygenation. -

Duodenal Expression of Iron Transport Molecules in Untreated
953 SMALL INTESTINE Duodenal expression of iron transport molecules in Gut: first published as 10.1136/gut.52.7.953 on 1 July 2003. Downloaded from untreated haemochromatosis subjects K A Stuart, G J Anderson, D M Frazer, L W Powell, M McCullen, L M Fletcher, D H G Crawford ............................................................................................................................. Gut 2003;52:953–959 Background and aims: In HFE associated hereditary haemochromatosis, the duodenal enterocyte behaves as if iron deficient and previous reports have shown increased duodenal expression of diva- lent metal transporter 1 (DMT1) and iron regulated gene 1 (Ireg1) in affected subjects. In those studies, many patients had undergone venesection, which is a potent stimulus of iron absorption. Our study investigated duodenal expression of DMT1 (IRE and non-IRE), Ireg1, hephaestin, and duodenal cytochrome-b(Dyctb) in untreated C282Y homozygous haemochromatosis patients, iron deficient patients, and iron replete subjects. See end of article for Methods: Total RNA was extracted from duodenal biopsies and expression of the iron transport genes authors’ affiliations was assessed by ribonuclease protection assay. ....................... Results: Expression of DMT1 (IRE) and Ireg1 was increased 3–5-fold in iron deficient subjects com- - Correspondence to: pared with iron replete subjects. Duodenal expression of DMT1 (IRE) and Ireg1 was similar in haemo Dr K Stuart, Department of chromatosis patients and iron replete subjects but in haemochromatosis patients with elevated serum Gastroenterology and ferritin concentrations, both DMT1 (IRE) and Ireg1 expression were inappropriately increased relative Hepatology, Princess to serum ferritin concentration. Hephaestin and Dcytb levels were not upregulated in haemochromato- Alexandra Hospital, sis. DMT1 (IRE) and Ireg1 levels showed significant inverse correlations with serum ferritin concentra- Ipswich Rd, Woolloongabba, 4102 tion in each group of patients. -

A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders
medicines Review A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders Andronicos Yiannikourides 1 and Gladys O. Latunde-Dada 2,* 1 Faculty of Life Sciences and Medicine, Henriette Raphael House Guy’s Campus King’s College London, London SE1 1UL, UK 2 Department of Nutritional Sciences, School of Life Course Sciences, King’s College London, Franklin-Wilkins-Building, 150 Stamford Street, London SE1 9NH, UK * Correspondence: [email protected] Received: 30 June 2019; Accepted: 2 August 2019; Published: 5 August 2019 Abstract: Iron is a vital trace element for humans, as it plays a crucial role in oxygen transport, oxidative metabolism, cellular proliferation, and many catalytic reactions. To be beneficial, the amount of iron in the human body needs to be maintained within the ideal range. Iron metabolism is one of the most complex processes involving many organs and tissues, the interaction of which is critical for iron homeostasis. No active mechanism for iron excretion exists. Therefore, the amount of iron absorbed by the intestine is tightly controlled to balance the daily losses. The bone marrow is the prime iron consumer in the body, being the site for erythropoiesis, while the reticuloendothelial system is responsible for iron recycling through erythrocyte phagocytosis. The liver has important synthetic, storing, and regulatory functions in iron homeostasis. Among the numerous proteins involved in iron metabolism, hepcidin is a liver-derived peptide hormone, which is the master regulator of iron metabolism. This hormone acts in many target tissues and regulates systemic iron levels through a negative feedback mechanism. Hepcidin synthesis is controlled by several factors such as iron levels, anaemia, infection, inflammation, and erythropoietic activity.