Thyrotoxicosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
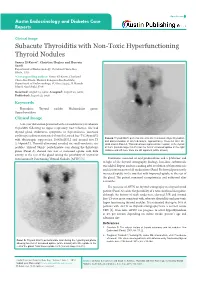
Subacute Thyroiditis with Non-Toxic Hyperfunctioning Thyroid Nodules
Open Access Austin Endocrinology and Diabetes Case Reports Clinical Image Subacute Thyroiditis with Non-Toxic Hyperfunctioning Thyroid Nodules Samer El-Kaissi*, Christine Hughes and Hussein Saadi Department of Endocrinology, Cleveland Clinic Abu Dhabi, UAE *Corresponding author: Samer El-Kaissi, Cleveland Clinic Abu Dhabi, Medical Subspecialties Institute, Department of Endocrinology, PO Box 112412, Al Maryah Island, Abu Dhabi, UAE Received: August 15, 2016; Accepted: August 22, 2016; Published: August 23, 2016 Keywords Thyroiditis; Thyroid nodule; Multinodular goitre; Hyperthyroidism Clinical Image A 46-year old woman presented with a 4-week history of subacute Thyroiditis following an upper respiratory tract infection. She had thyroid gland tenderness, symptoms of thyrotoxicosis, increased erythrocyte sedimentation rate [45mm/hr], raised free-T4 [29pmol/L] Panel B: Thyroid 99mTc pertechnetate scan after resolution of hyperthyroidism with thyrotropin suppression [0.005mIU/L] and normal free-T3 and discontinuation of all medications, approximately 10-weeks after the [5.64pmol/L]. Thyroid ultrasound revealed six small-moderate size initial scan in Panel A. This scan shows improved tracer uptake in the thyroid nodules. Thyroid 99mTc pertechnetate scan during the thyrotoxic at 0.6% (normal range 0.2-3%) but the foci of increased uptake in the right phase (Panel A) showed two foci of increased uptake with little midzone and left lower zone are still apparent (white arrows). activity in the rest of the gland raising the possibility of coexistent Autonomously Functioning Thyroid Nodules [AFTN] [1]. Treatment consisted of oral prednisolone and a β-blocker and in light of the thyroid scintigraphy findings, low-dose carbimazole was added. Repeat nuclear scanning after resolution of thyrotoxicosis and discontinuation of all medications (Panel B) showed persistently increased uptake in the two foci with improved uptake in the rest of the gland. -

The Subacute Thyroiditis, Since Its First Description by De Quervain (1904), Has Been Reported Under Many Synonyms
Endocrinol. Japon. 1964, 11 (2), 119~138 STUDIES ON SUBACUTE THYROIDITIS SHOZO SAITO Department of Radiology, School of Medicine, Gunma University, Maebashi The subacute thyroiditis, since its first description by de Quervain (1904), has been reported under many synonyms. Terms given with reference to the histo- logic features of this disease include "pseudotuberculous thyroiditis", "granulo- matous thyroiditis", "gaint-cell thyroiditis", "de Quervain's thyroiditis", "struma granulomatosa" or "struma fibrosa, giant cell variant". The clinical findings have given rise to such names as" acute and subacute nonsuppurative thyroiditis", "acute noninfectious thyroiditis"and"subacute thyroiditis" . In recent writings, the disease is most commonly referred to as subacute thyroiditis. Later, de Quervain and Giordanengo (1936) collected reports of 54 cases of the disease, appearing in the literature since de Quervain's original description, and clearly distinguished it from other forms of thyroiditis. Several years ago, the disease had been considered relatively rare, but with recent advance in endocrinology, more attentions have been drawn by it, and more reports published on it. For the past six years, the author has treated 51 cases of subacute thyroiditis, and obtained findings of interest, which are outlined in this paper. METHODS OF EXAMINATION The disease was confirmed by Silverman's needle biopsy (Crile and Rumsey, 1950; Crile and Hazard, 1951) of the thyroid in 36 of them, and the others were diagnosed by typical clinical symptoms, characteristic results of thyroidal function test and accelerated red cell sedimentation rate and its clinical course. Thyroid function test Basal metabolic rate (BMR) was examined by Knipping's method (normal range•}15%), and serum protein-bound iodine (PBI) by modification of Barker's method (Barker and Humphrey, 1950). -

Disease/Medical Condition
Disease/Medical Condition HYPOTHYROIDISM Date of Publication: January 27, 2017 (also known as “underactive thyroid disease”; includes congenital hypothyroidism [also known as “neonatal hypothyroidism”] and Hashimoto’s thyroiditis [also known as “autoimmune thyroiditis”]; may manifest as “cretinism” [if onsets during fetal or early life; also known as “congenital myxedema”] or “myxedema” [if onset occurs in older children and adults]) Is the initiation of non-invasive dental hygiene procedures* contra-indicated? No. ◼ Is medical consult advised? – Yes, if previously undiagnosed hypothyroidism or enlarged (or shrunken) thyroid gland is suspected1, in which case the patient/client should see his/her primary care physician. Detection early in childhood can prevent permanent intellectual impairment. – Yes, if previously diagnosed hypothyroidism is suspected to be undermedicated (with manifest signs/symptoms of hypothyroidism) or overmedicated (with manifest signs/symptoms of hyperthyroidism2), in which case the patient/client should see his/her primary care physician or endocrinologist. Major stress or illness sometimes necessitates an increase in prescribed thyroid hormone. Is the initiation of invasive dental hygiene procedures contra-indicated?** Possibly, depending on the certainty of diagnosis and level of control. ◼ Is medical consult advised? – See above. ◼ Is medical clearance required? – Yes, if undiagnosed or severe hypothyroidism is suspected. ◼ Is antibiotic prophylaxis required? – No. ◼ Is postponing treatment advised? – Yes, if undiagnosed hypothyroidism is suspected (necessitating medical assessment/management) or severe hypothyroidism is suspected (necessitating urgent medical assessment/management in order to avoid risk of myxedema coma). In general, the patient/client with mild symptoms of untreated hypothyroidism is not in danger when receiving dental hygiene therapy, and the well managed (euthyroid) patient/client requires no special regard. -

Myopathy in Acute Hypothyroidism
Postgrad Med J: first published as 10.1136/pgmj.63.742.661 on 1 August 1987. Downloaded from Postgraduate Medical Journal (1987) 63, 661-663 Clinical Reports Myopathy in acute hypothyroidism A.W.C. Kung, J.T.C. Ma, Y.L. Yu, C.C.L. Wang, E.K.W. Woo, K.S.L. Lam, C.Y. Huang and R.T.T. Yeung Department ofMedicine, University ofHong Kong, Queen Mary Hospital, Pokfulam Road, Hong Kong. Summary: Hypothyroid myopathy has so far been reported in long standing cases ofhypothyroidism. We describe two adult patients with myopathy associated with acute transient hypothyroidism. Both presented with severe muscle aches and cramps, stiffness and spasms. Muscle enzymes were markedly elevated and electromyography in one patient showed myopathic features. Histological changes were absent in muscle biopsy, probably because of the short duration of metabolic disturbance. The myopathy subsided promptly when the hypothyroid state was reversed. Introduction Hypothyroid myopathy has been described in patients however no muscle weakness or symptoms of hypo- with long-standing hypothyroidism.' 3 We report two thyroidism. She had no preceding viral infection. Chinese patients with muscle involvement in acute Examination revealed that the goitre had decreased in copyright. transient hypothyroidism, who improved after rever- size and she was clinically euthyroid. Motor and sal of the hypothyroid state, sensory functions were completely normal. Investigation showed a T4 of 44nmol/l, triiodo- thyronine (T3) 1.2 nmol/I (normal 0.77-2.97 nmol/1), Case reports and TSH 5.1 AIU/ml (normal up to 7). The serum creatine kinase level was 11,940ILmol/min.l (normal Case I 5-165) and the lactic dehydrogenase was 397;Lmol/ min.l (normal 130-275), and glutamic-oxalacetic A 42 year old woman presented at another hospital transaminase was 120limol/min.l (normal 28-32). -

SUBACUTE THYROIDITIS by SELWYN TAYLOR, M.CH., F.R.C.S
Postgrad Med J: first published as 10.1136/pgmj.33.381.327 on 1 July 1957. Downloaded from 327 SUBACUTE THYROIDITIS By SELWYN TAYLOR, M.CH., F.R.C.S. Surgeon, King's College Hospital, Belgrave Hospitalfor Children, and Hammersmith Hospital; Lecturer in Surgery, Postgraduate Medical School of London The term ' thyroiditis ' implies inflammation of to become much more common in that particular the thyroid gland, but by long usage it has come clinic. I had never seen an example before I950, to be used for a number of conditions in which but saw six in the next three years and pro- infection or trauma play apparently no part. Sub- gressively more each year since then. The con- acute thyroiditis is the title given to a condition dition is much commoner in women than men in which was first clearly described by de Quervain the ratio of about six to one. It has not yet been in I904 and which has been rediscovered, or re- reported in a child and is commonest in the fourth described, on a number of occasions since then, and fifth decades, although I have seen it in a with the result that it now has a multiplicity of student teacher of twenty-one. In our own series different names: granulomatous thyroiditis, giant- there was a history of a pre-existing goitre in 50 cell thyroiditis, pseudotuberculous thyroiditis, per cent. of the patients. The incidence, compared creeping thyroiditis, struma granulomatosa, acute with that of Hashimoto's thyroiditis and Riedel's non-infectious thyroiditis, acute non-suppurative thyroiditis, varies widely in different clinics, but thyroiditis and de Quervain's thyroiditis. -

Screening for Thyroid Dysfunction: U.S. Preventive Services Task Force Recommendation Statement Michael L
Annals of Internal Medicine CLINICAL GUIDELINE Screening for Thyroid Dysfunction: U.S. Preventive Services Task Force Recommendation Statement Michael L. LeFevre, MD, MSPH, on behalf of the U.S. Preventive Services Task Force* Description: Update of the 2004 U.S. Preventive Services Task Recommendation: The USPSTF concludes that the current ev- Force (USPSTF) recommendation on screening for thyroid idence is insufficient to assess the balance of benefits and harms disease. of screening for thyroid dysfunction in nonpregnant, asymptom- atic adults. (I statement) Methods: The USPSTF reviewed the evidence on the benefits and harms of screening for subclinical and “overt” thyroid dysfunction without clinically obvious symptoms, as well as the Ann Intern Med. 2015;162:641-650. doi:10.7326/M15-0483 www.annals.org effects of treatment on intermediate and final health outcomes. For author affiliation, see end of text. * For a list of USPSTF members, see the Appendix (available at www.annals Population: This recommendation applies to nonpregnant, .org). asymptomatic adults. This article was published online first at www.annals.org on 24 March 2015. he U.S. Preventive Services Task Force (USPSTF) clinicians. Thyroid dysfunction represents a continuum Tmakes recommendations about the effectiveness of from asymptomatic biochemical changes to clinically specific preventive care services for patients without re- symptomatic disease. In rare cases, it can produce life- lated signs or symptoms. threatening complications, such as myxedema coma or It bases its recommendations on the evidence of thyroid storm (1, 2). both the benefits and harms of the service and an as- Subclinical hypothyroidism is defined as an asymp- sessment of the balance. -
Care Step Pathway – Thyroiditis (Inflammation of the Thyroid Gland)
Care Step Pathway – Thyroiditis (inflammation of the thyroid gland) Assessment Look: Listen: Recognize: - Appear unwell? - Appetite/weight changes? - Other immune-related toxicity? - Changes in weight since last visit? - Hot or cold intolerance? - Prior thyroid dysfunction? o Appear heavier? Thinner? - Change in energy, mood, or behavior? - Prior history of radiation therapy? - Changes in hair texture/thickness? - Palpitations? - Signs of thyroid storm (fever, tachycardia, sweating, dehydration, cardiac - Appear hot/cold? - Increased fatigue? decompensation, delirium/psychosis, liver failure, abdominal pain, - Look fatigued? - Bowel-related changes? nausea/vomiting, diarrhea) - Sweating? Constipation/diarrhea - Hyperactive or lethargic? o - Signs of airway compression - Difficulty breathing? - Shortness of breath/edema? - Clinical presentation: Occasionally thyroiditis with transient hyperthyroidism - Swollen neck? - Skin-related changes? (low TSH and high free T4) may be followed by more longstanding - Voice change (e.g., deeper voice) o Dry/oily hypothyroidism (high TSH and low free T4) - Differential diagnosis-- Primary hypothyroidism: High TSH with low free T4; secondary (central) hypothyroidism due to hypophysitis: both TSH and free T4 are low (see HCP Assessment below for more detail about testing) Grading Toxicity HYPOTHYROIDISM Definition: A disorder characterized by decreased production of thyroid hormones from the thyroid gland Asymptomatic, subclinical Asymptomatic, subclinical Symptomatic, primary Severely symptomatic, Life-threatening, -

Endocrine Emergencies
Endocrine Emergencies • Neuroendocrine response to Critical illness • Thyroid storm/Myxedema Coma • Adrenal Crisis/Sepsis • Hyper/Hypocalcemia • Hypoglycemia • Hyper and Hyponatremia • Pheochromocytoma crises CASE 76 year old man presents with urosepsis and is Admitted to MICU. He has chronic renal insufficiency. During his hospital course, he is intubated and treated With dopamine. Thyroid studies are performed for Inability to wean from ventilator. What labs do you want? Assessment of Thyroid Function • Hormone Levels: Total T4, Total T3 • Binding proteins: TBG, (T3*) Resin uptake • Free Hormone Levels: TSH, F T4, F T3, Free Thyroid Index, F T4 by Eq Dialysis • Radioactive Iodine uptake (RAIU); primarily for DDx of hyperthyroidism • Thyroid antibodies; TPO, Anti-Thyroglobulin, Thyroid stimulating immunoglobulins, Th receptor antibodies Labs: T4 2.4 ug/dl (5-12) T3U 40% (25-35) FTI 1.0 (1.2-4.2) FT4 0.6 (0.8-1.8) TSH 0.2 uU/ml (.4-5.0) Non-thyroidal illness • Hypothesis: NTI vs 2° Hypothyroidism –RT3 ↑ in NTI and ↓ in Hypothyroidism • Hypothesis: NTI vs Hyperthyroidism – TT3 ↓ in NTI and in ↑ Hyperthyroidism • 75 year old woman with history of hypothyroidism is found unresponsive in her home during a cold spell in houston. No heat in the home. • Exam: T° 95, BP 100/60, P 50, RR 8 • Periorbital edema, neck scar, no rub or gallop, distant heart sounds, crackles at bases, peripheral edema • ECG: Decreased voltage, runs of Torsade de pointes • Labs? Imaging? • CXR: cardiomegaly • Glucose 50 • Na+ 120, K+ 4, Cl 80, HCO3¯ 30 • BUN 30 Creat 1.4 • ABG: pH 7.25, PCO2 75, PO2 80 • CK 600 • Thyroid studies pending • Management: Manifestations of Myxedema Coma • Precipitated by infection, iatrogenic (surgery, sedation, diuretics) • Low thyroid studies • Hypothermia • Altered mental status • Hyponatremia • ↑pCO2 • ↑CK • ↑Catecholamines with ↑vascular resistance • Cardiac: low voltage, Pericardial effusion, impaired relaxation with ↓C.O. -

Subacute Thyroiditis Christa M
ENDOCRINECONSULT Subacute Thyroiditis Christa M. Blose, MPAS, PA-C, Holly Jodon, MPAS, PA-C erry, a 48-year-old white man, is referred to endocri- TABLE Jnology for abnormal results Lab Results for Case Patient of thyroid tests performed four Time after symptom onset weeks ago (see table for values). Two months ago, Jerry developed 1 mo 2 mo 4 mo 6 mo an upper respiratory infection TSH (0.40-4.50 mlU/mL) 0.03 0.14 8.23 3.54 (URI) with fever, odynophagia, and anterior neck discomfort. Free T4 (0.8-1.8 ng/dL) 2.58 1.74 0.72 1.66 His symptoms resolved after two weeks; however, he has since de- Free T3 (2.3-4.2 pg/mL) 4.12 1.7 3.0 veloped fatigue and nervousness. The remaining review of sys- TPO antibody (< 20) 11 tems is unremarkable. Medical ESR (0-22 mm/h) 55 history is negative. Jerry denies any factors that can affect thyroid Abbreviations: ESR, erythrocyte sedimentation rate; T3, triiodothyronine; T4, thyroxine; function: He does not take thyroid TPO, thyroid peroxidase antibody. medication, OTC thyroid supple- ments, amiodarone, lithium, or TSH, with normal free thyroxine except for a small, firm thyroid interferon-α, does not have high (T4) and free triiodothyronine gland without the tenderness iodine intake, and has not un- (T3) levels. His thyroid peroxidase elicited previously. Labwork re- dergone head/neck irradiation. antibody (Anti-TPO) is negative. veals an elevated TSH with low There is no personal or family Radioactive iodine uptake (RAIU) free T4 and free T3. -

Thyroid Emergencies
The Art and Science of Infusion Nursing Angela M. Leung , MD, MSc Thyroid Emergencies cal manifestations, diagnostic methods, and treatments of ABSTRACT hypothyroidism and hyperthyroidism, both in the Myxedema coma and thyroid storm are thyroid nonacute and life-threatening forms of these diseases. emergencies associated with increased mortality. Prompt recognition of these states—which repre- sent the severe, life-threatening conditions of THYROID HORMONE PRODUCTION AND METABOLISM extremely reduced or elevated circulating thyroid hormone concentrations, respectively—is neces- sary to initiate treatment. Management of myxe- The normal physiology of the hypothalamic-pituitary- dema coma and thyroid storm requires both med- thyroid axis involves the production of T4 and T3 by the thyroid gland, a process that is regulated by thyroid- ical and supportive therapies and should be treated stimulating hormone (TSH) secreted by the pituitary, in an intensive care unit setting. which is, in turn, regulated by thyrotropin-releasing hor- Key words: hypothyroidism , hyperthyroidism , mone (TRH) secreted by the hypothalamus. Both serum ICU , myxedema coma , thyroid emergencies , T4 and T3 concentrations act as negative feedback regu- thyroid storm lators of TSH and TRH secretion, but can be altered by environmental conditions—including food availability he thyroid is a 15- to 20-gram gland located and temperature—and disease states, such as infection. 1 in the anterior neck. It is responsible for the Thyroid hormone synthesis is achieved first through production of the thyroid hormones T4 (thy- active transport of circulating iodide, which is taken in roxine) and T3 (triiodothyronine). Various from the diet, by the sodium/iodide symporter located at factors can affect thyroid hormone synthesis, the basolateral membrane of the thyroid follicular cell. -

Hypothyroid Face
Hypothyroidism - Signs and Symptoms Classic Teaching Symptoms % Symptoms % Symptoms % Weakness 99 Thick tongue 82 Dyspnea 55 Dry skin 97 Facial edema 79 Peripheral edema 55 Coarse skin 97 Coarse hair 76 Hoarseness 52 Lethargy 91 Skin pallor 67 Anorexia 45 Slow speech 91 Memory loss 66 Nervousness 35 Eyelid edema 90 Constipation 61 Menorrhagia 32 Feeling cold 89 Weight gain 59 Palpitations 31 Less sweating 89 Hair loss 57 Deafness 30 Cold skin 83 Lip pallor 57 Precordial pain 25 Galactorrhea ? modified from Means, 1948 Hypothyroid Face Notice the apathetic facies, bilateral ptosis, and absent eyebrows Faces of Clinical Hypothyroidism Frequency of Cutaneous Findings in Hypothyroidism* Cutaneous Manifestations Frequency (%) Cold intolerance 50-95 Thickening & dryness of hair & skin 80-90 Edema of hands, face, and/or eyelids 70-85 Malar flush 55 Pitting-dependent edema 30 Alopecia (loss or thinning of hair) 30-40 Eyebrows 25 Scalp 20 Pallor 25-60 Yellow tint to skin 25-50 Decrease or loss of sweating 10-70 *modified from Freedberg and Vogel in Werner’s and Ingbar’s The Thyroid 6th ed. Delayed Deep Tendon Reflex in Hypothyroidism • Achilles’ tendon reflex time most commonly sought but may also be effectively tested on brachioradialis or biceps • Achilles’ tendon reflex Hypothyroid timing is best elicited with patient kneeling TIME • Intensity of hammer percussion should be the lightest possible stroke that evokes reflex Normal Graves' Disease Goiter Hyperthyroidism Exophthalmos Localized myxedema Thyroid acropachy Thyroid stimulating -

Subacute Thyroiditis: Diagnostic Difficulties and Simple Treatment
SUBACUTE THYROIDITIS: DIAGNOSTIC DIFFICULTIES AND SIMPLE TREATMENT Joel I. Hamburger Northland Thyroid Laboratory, Southfield, Michigan Subacute thyroiditis (SAT) constitutes 0.8% produce the systemic manifestations of hyperthyroid of referrals to Northland Thyroid Laboratory ism, usually mild. and is one tenth as common as hyperthyroidism. The laboratory abnormalities are readily under The peak age is 30—SOyears, and women pre standable in terms of the underlying pathophysiology. dominated by a factor of 4.5. Two thirds of the As a consequence of the diffuse inflammation, the patients presented in typical fashion with a thyroidal uptake of radioactive iodine (RAI) is im painful tender goiter, but for one third the paired (1—9). The discharge of thyroid hormone presentation was atypical in that there was no elevates serum thyroxine values (ST4) (1—9). The pain, the principal complaint in most cases erythrocyte sedimentation rate (ESR) is elevated being painless goiter, thyroid nodule, or fea as a nonspecific response to inflammation (1—9), and tures of hyperthyroidism. Elevated erythrocyte occasionally there is an increase in the white blood sedimentation rates were found in all typical cell count (WBC) as well (3,6—9). patients and in 11 of 14 atypical patients. As the disease progresses the local and systemic Serum thyroxine iodide values were elevated in features decline until full recovery is realized, usually two thirds of the patients, typical and atypi within 2—4months. In some instances there is a cal. The radioactive iodine uptake was sub temporary hypothyroid phase (2,3) which rarely nornwl for all and responded subnormally to may prove permanent (10) .