Snrpg (NM 026506) Mouse Tagged ORF Clone – MR220563 | Origene
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unrip (STRAP) (NM 007178) Human Tagged ORF Clone Lentiviral Particle Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for RC209149L3V Unrip (STRAP) (NM_007178) Human Tagged ORF Clone Lentiviral Particle Product data: Product Type: Lentiviral Particles Product Name: Unrip (STRAP) (NM_007178) Human Tagged ORF Clone Lentiviral Particle Symbol: STRAP Synonyms: MAWD; PT-WD; UNRIP Vector: pLenti-C-Myc-DDK-P2A-Puro (PS100092) ACCN: NM_007178 ORF Size: 1050 bp ORF Nucleotide The ORF insert of this clone is exactly the same as(RC209149). Sequence: OTI Disclaimer: The molecular sequence of this clone aligns with the gene accession number as a point of reference only. However, individual transcript sequences of the same gene can differ through naturally occurring variations (e.g. polymorphisms), each with its own valid existence. This clone is substantially in agreement with the reference, but a complete review of all prevailing variants is recommended prior to use. More info OTI Annotation: This clone was engineered to express the complete ORF with an expression tag. Expression varies depending on the nature of the gene. RefSeq: NM_007178.2 RefSeq Size: 1924 bp RefSeq ORF: 1053 bp Locus ID: 11171 UniProt ID: Q9Y3F4 Domains: WD40 Protein Families: Druggable Genome MW: 38.4 kDa This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 2 Unrip (STRAP) (NM_007178) Human Tagged ORF Clone Lentiviral Particle – RC209149L3V Gene Summary: The SMN complex plays a catalyst role in the assembly of small nuclear ribonucleoproteins (snRNPs), the building blocks of the spliceosome. -

Supplementary Materials
Supplementary Materials COMPARATIVE ANALYSIS OF THE TRANSCRIPTOME, PROTEOME AND miRNA PROFILE OF KUPFFER CELLS AND MONOCYTES Andrey Elchaninov1,3*, Anastasiya Lokhonina1,3, Maria Nikitina2, Polina Vishnyakova1,3, Andrey Makarov1, Irina Arutyunyan1, Anastasiya Poltavets1, Evgeniya Kananykhina2, Sergey Kovalchuk4, Evgeny Karpulevich5,6, Galina Bolshakova2, Gennady Sukhikh1, Timur Fatkhudinov2,3 1 Laboratory of Regenerative Medicine, National Medical Research Center for Obstetrics, Gynecology and Perinatology Named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation, Moscow, Russia 2 Laboratory of Growth and Development, Scientific Research Institute of Human Morphology, Moscow, Russia 3 Histology Department, Medical Institute, Peoples' Friendship University of Russia, Moscow, Russia 4 Laboratory of Bioinformatic methods for Combinatorial Chemistry and Biology, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences, Moscow, Russia 5 Information Systems Department, Ivannikov Institute for System Programming of the Russian Academy of Sciences, Moscow, Russia 6 Genome Engineering Laboratory, Moscow Institute of Physics and Technology, Dolgoprudny, Moscow Region, Russia Figure S1. Flow cytometry analysis of unsorted blood sample. Representative forward, side scattering and histogram are shown. The proportions of negative cells were determined in relation to the isotype controls. The percentages of positive cells are indicated. The blue curve corresponds to the isotype control. Figure S2. Flow cytometry analysis of unsorted liver stromal cells. Representative forward, side scattering and histogram are shown. The proportions of negative cells were determined in relation to the isotype controls. The percentages of positive cells are indicated. The blue curve corresponds to the isotype control. Figure S3. MiRNAs expression analysis in monocytes and Kupffer cells. Full-length of heatmaps are presented. -

Immune Adaptation to Chronic Intense Exercise Training: New Microarray Evidence Dongmei Liu1†, Ru Wang1†, Ana R
Liu et al. BMC Genomics (2017) 18:29 DOI 10.1186/s12864-016-3388-5 RESEARCHARTICLE Open Access Immune adaptation to chronic intense exercise training: new microarray evidence Dongmei Liu1†, Ru Wang1†, Ana R. Grant2, Jinming Zhang3, Paul M. Gordon4, Yuqin Wei1 and Peijie Chen1* Abstract Background: Endurance exercise training, especially the high-intensity training, exhibits a strong influence on the immune system. However, the mechanisms underpinning the immune-regulatory effect of exercise remain unclear. Consequently, we chose to investigate the alterations in the transcriptional profile of blood leukocytes in young endurance athletes as compared with healthy sedentary controls, using Affymetrix human gene 1.1 ST array. Results: Group differences in the transcriptome were analyzed using Intensity-based Hierarchical Bayes method followed by a Logistic Regression-based gene set enrichment method. We identified 72 significant transcripts differentially expressed in the leukocyte transcriptome of young endurance athletes as compared with non-athlete controls with a false discovery rate (FDR) < 0.05, comprising mainly the genes encoding ribosomal proteins and the genes involved in mitochondrial oxidative phosphorylation. Gene set enrichment analysis identified three major gene set clusters: two were up-regulated in athletes including gene translation and ribosomal protein production, and mitochondria oxidative phosphorylation and biogenesis; one gene set cluster identified as transcriptionally downregulated in athletes was related to inflammation and immune activity. Conclusion: Our data indicates that in young healthy individuals, intense endurance exercise training (exemplifed by athletic training) can chronically induce transcriptional changes in the peripheral blood leukocytes, upregulating genes related to protein production and mitochondrial energetics, and downregulating genes involved in inflammatory response. -

U1 Snrnp Regulates Chromatin Retention of Noncoding Rnas
bioRxiv preprint doi: https://doi.org/10.1101/310433; this version posted April 29, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. U1 snRNP regulates chromatin retention of noncoding RNAs Yafei Yin,1,* J. Yuyang Lu,1 Xuechun Zhang,1 Wen Shao,1 Yanhui Xu,1 Pan Li,1,2 Yantao Hong,1 Qiangfeng Cliff Zhang,2 and Xiaohua Shen 1,3,* 1 Tsinghua-Peking Center for Life Sciences, School of Medicine and School of Life Sciences, Tsinghua University, Beijing 100084, China 2 MOE Key Laboratory of Bioinformatics, Beijing Advanced Innovation Center for Structural Biology, Center for Synthetic and Systems Biology, School of Life Sciences, Tsinghua University, Beijing 100084, China 3 Lead Contact *Correspondence: [email protected] (Y.Y.), [email protected] (X.S.) Running title: U1 snRNP regulates ncRNA-chromatin retention Keywords: RNA-chromatin localization, U1 snRNA, lncRNA, PROMPTs and eRNA, splicing 1 bioRxiv preprint doi: https://doi.org/10.1101/310433; this version posted April 29, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Thousands of noncoding transcripts exist in mammalian genomes, and they preferentially localize to chromatin. Here, to identify cis-regulatory elements that control RNA-chromatin association, we developed a high-throughput method named RNA element for subcellular localization by sequencing (REL-seq). Coupling REL-seq with random mutagenesis (mutREL-seq), we discovered a key 7-nt U1 recognition motif in chromatin-enriched RNA elements. -

Anti-SNRPD3 Antibody (ARG55763)
Product datasheet [email protected] ARG55763 Package: 100 μl anti-SNRPD3 antibody Store at: -20°C Summary Product Description Rabbit Polyclonal antibody recognizes SNRPD3 Tested Reactivity Hu Predict Reactivity Ms, Xenopus Tested Application FACS, IHC-P, WB Host Rabbit Clonality Polyclonal Isotype IgG Target Name SNRPD3 Antigen Species Human Immunogen KLH-conjugated synthetic peptide corresponding to aa. 99-126 (C-terminus) of Human SNRPD3. Conjugation Un-conjugated Alternate Names Small nuclear ribonucleoprotein Sm D3; Sm-D3; snRNP core protein D3; SMD3 Application Instructions Application table Application Dilution FACS 1:10 - 1:50 IHC-P 1:50 - 1:100 WB 1:1000 Application Note * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. Positive Control MCF7 Calculated Mw 14 kDa Properties Form Liquid Purification Purification with Protein A and immunogen peptide. Buffer PBS and 0.09% (W/V) Sodium azide. Preservative 0.09% (W/V) Sodium azide. Storage instruction For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C or below. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. www.arigobio.com 1/3 Note For laboratory research only, not for drug, diagnostic or other use. Bioinformation Database links GeneID: 6634 Human Swiss-port # P62318 Human Gene Symbol SNRPD3 Gene Full Name small nuclear ribonucleoprotein D3 polypeptide 18kDa Background This gene encodes a core component of the spliceosome, which is a nuclear ribonucleoprotein complex that functions in pre-mRNA splicing. -

Downregulation of SNRPG Induces Cell Cycle Arrest and Sensitizes Human Glioblastoma Cells to Temozolomide by Targeting Myc Through a P53-Dependent Signaling Pathway
Cancer Biol Med 2020. doi: 10.20892/j.issn.2095-3941.2019.0164 ORIGINAL ARTICLE Downregulation of SNRPG induces cell cycle arrest and sensitizes human glioblastoma cells to temozolomide by targeting Myc through a p53-dependent signaling pathway Yulong Lan1,2*, Jiacheng Lou2*, Jiliang Hu1, Zhikuan Yu1, Wen Lyu1, Bo Zhang1,2 1Department of Neurosurgery, Shenzhen People’s Hospital, Second Clinical Medical College of Jinan University, The First Affiliated Hospital of Southern University of Science and Technology, Shenzhen 518020, China;2 Department of Neurosurgery, The Second Affiliated Hospital of Dalian Medical University, Dalian 116023, China ABSTRACT Objective: Temozolomide (TMZ) is commonly used for glioblastoma multiforme (GBM) chemotherapy. However, drug resistance limits its therapeutic effect in GBM treatment. RNA-binding proteins (RBPs) have vital roles in posttranscriptional events. While disturbance of RBP-RNA network activity is potentially associated with cancer development, the precise mechanisms are not fully known. The SNRPG gene, encoding small nuclear ribonucleoprotein polypeptide G, was recently found to be related to cancer incidence, but its exact function has yet to be elucidated. Methods: SNRPG knockdown was achieved via short hairpin RNAs. Gene expression profiling and Western blot analyses were used to identify potential glioma cell growth signaling pathways affected by SNRPG. Xenograft tumors were examined to determine the carcinogenic effects of SNRPG on glioma tissues. Results: The SNRPG-mediated inhibitory effect on glioma cells might be due to the targeted prevention of Myc and p53. In addition, the effects of SNRPG loss on p53 levels and cell cycle progression were found to be Myc-dependent. Furthermore, SNRPG was increased in TMZ-resistant GBM cells, and downregulation of SNRPG potentially sensitized resistant cells to TMZ, suggesting that SNRPG deficiency decreases the chemoresistance of GBM cells to TMZ via the p53 signaling pathway. -

Downloaded from the TCGA Data Portal Website ( Cell Proliferation Was Determined Using an MTT Assay
ORIGINAL RESEARCH published: 28 August 2020 doi: 10.3389/fonc.2020.01468 Clinical Significance, Cellular Function, and Potential Molecular Pathways of CCT7 in Endometrial Cancer Liwen Wang 1,2†, Wei Zhou 1†, Hui Li 2,3, Hui Yang 1 and Nianchun Shan 1* 1 Department of Gynecology and Obstetrics, Xiangya Hospital, Central South University, Changsha, China, 2 Xiangya School of Medicine, Central South University, Changsha, China, 3 Department of Reproductive, Xiangya Hospital, Central South University, Changsha, China Objective: Endometrial cancer (EC) is a common gynecologic malignancy; myometrial Edited by: invasion (MI) is a typical approach of EC spreads and an important index to assess tumor Sarah M. Temkin, metastasis and outcome in EC patients. CCT7 is a member of the TCP1 chaperone Virginia Commonwealth University, family, involved in cytoskeletal protein folding and unfolding. In this study, the role of United States CCT7 in EC development was investigated. Reviewed by: Syed S. Islam, Methods: Clinical data for 87 EC cases and expression of CCT7 were analyzed. CCT7 King Faisal Specialist Hospital & Research Centre, Saudi Arabia was knocked out using siRNA-CCT7 in Ishikawa and RL95-2 cells, and their function Elena Gershtein, about proliferation, apoptosis, and invasion was further tested. Bioinformatics methods Russian Cancer Research Center NN were used to predict the potential pathways of CCT7 in EC development. Blokhin, Russia *Correspondence: Results: The rates of CCT7-positive cells in EC and adjacent normal endometrium Nianchun Shan tissues had a significant difference (67.8 vs. 51.4%, p = 0.035), and the expression [email protected] rate increased from low to high pathological stage (39.7% in the I/II stage, 71.4% in the †These authors have contributed III/IV stage, p = 0.029). -
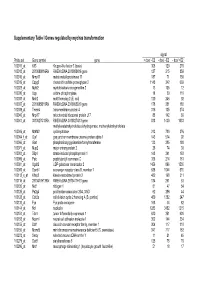
Supplementary Table I Genes Regulated by Myc/Ras Transformation
Supplementary Table I Genes regulated by myc/ras transformation signal Probe set Gene symbol gene + dox/ - E2 - dox/ - E2 - dox/ +E2 100011_at Klf3 Kruppel-like factor 3 (basic) 308 120 270 100013_at 2010008K16Rik RIKEN cDNA 2010008K16 gene 127 215 859 100016_at Mmp11 matrix metalloproteinase 11 187 71 150 100019_at Cspg2 chondroitin sulfate proteoglycan 2 1148 342 669 100023_at Mybl2 myeloblastosis oncogene-like 2 13 105 12 100030_at Upp uridine phosphorylase 18 39 110 100033_at Msh2 mutS homolog 2 (E. coli) 120 246 92 100037_at 2310005B10Rik RIKEN cDNA 2310005B10 gene 178 351 188 100039_at Tmem4 transmembrane protein 4 316 135 274 100040_at Mrpl17 mitochondrial ribosomal protein L17 88 142 89 100041_at 3010027G13Rik RIKEN cDNA 3010027G13 gene 818 1430 1033 methylenetetrahydrofolate dehydrogenase, methenyltetrahydrofolate 100046_at Mthfd2 cyclohydrolase 213 720 376 100064_f_at Gja1 gap junction membrane channel protein alpha 1 143 574 92 100066_at Gart phosphoribosylglycinamide formyltransferase 133 385 180 100071_at Mup2 major urinary protein 2 26 74 30 100081_at Stip1 stress-induced phosphoprotein 1 148 341 163 100089_at Ppic peptidylprolyl isomerase C 358 214 181 100091_at Ugalt2 UDP-galactose translocator 2 1456 896 1530 100095_at Scarb1 scavenger receptor class B, member 1 638 1044 570 100113_s_at Kifap3 kinesin-associated protein 3 482 168 311 100116_at 2810417H13Rik RIKEN cDNA 2810417H13 gene 134 261 53 100120_at Nid1 nidogen 1 81 47 54 100125_at Pa2g4 proliferation-associated 2G4, 38kD 62 286 44 100128_at Cdc2a cell division cycle 2 homolog A (S. pombe) 459 1382 247 100133_at Fyn Fyn proto-oncogene 168 44 82 100144_at Ncl nucleolin 1283 3452 1215 100151_at Tde1 tumor differentially expressed 1 620 351 620 100153_at Ncam1 neural cell adhesion molecule 1 302 144 234 100155_at Ddr1 discoidin domain receptor family, member 1 304 117 310 100156_at Mcmd5 mini chromosome maintenance deficient 5 (S. -

GSE50161, (C) GSE66354, (D) GSE74195 and (E) GSE86574
Figure S1. Boxplots of normalized samples in five datasets. (A) GSE25604, (B) GSE50161, (C) GSE66354, (D) GSE74195 and (E) GSE86574. The x‑axes indicate samples, and the y‑axes represent the expression of genes. Figure S2. Volanco plots of DEGs in five datasets. (A) GSE25604, (B) GSE50161, (C) GSE66354, (D) GSE74195 and (E) GSE86574. Red nodes represent upregulated DEGs and green nodes indicate downregulated DEGs. Cut‑off criteria were P<0.05 and |log2 FC|>1. DEGs, differentially expressed genes; FC, fold change; adj.P.Val, adjusted P‑value. Figure S3. Transcription factor‑gene regulatory network constructed using the Cytoscape iRegulion plug‑in. Table SI. Primer sequences for reverse transcription‑quantitative polymerase chain reaction. Genes Sequences hsa‑miR‑124 F: 5'‑ACACTCCAGCTGGGCAGCAGCAATTCATGTTT‑3' R: 5'‑CTCAACTGGTGTCGTGGA‑3' hsa‑miR‑330‑3p F: 5'‑CATGAATTCACTCTCCCCGTTTCTCCCTCTGC‑3' R: 5'‑CCTGCGGCCGCGAGCCGCCCTGTTTGTCTGAG‑3' hsa‑miR‑34a‑5p F: 5'‑TGGCAGTGTCTTAGCTGGTTGT‑3' R: 5'‑GCGAGCACAGAATTAATACGAC‑3' hsa‑miR‑449a F: 5'‑TGCGGTGGCAGTGTATTGTTAGC‑3' R: 5'‑CCAGTGCAGGGTCCGAGGT‑3' CD44 F: 5'‑CGGACACCATGGACAAGTTT‑3' R: 5'‑TGTCAATCCAGTTTCAGCATCA‑3' PCNA F: 5'‑GAACTGGTTCATTCATCTCTATGG‑3' F: 5'‑TGTCACAGACAAGTAATGTCGATAAA‑3' SYT1 F: 5'‑CAATAGCCATAGTCGCAGTCCT‑3' R: 5'‑TGTCAATCCAGTTTCAGCATCA‑3' U6 F: 5'‑GCTTCGGCAGCACATATACTAAAAT‑3' R: 5'‑CGCTTCACGAATTTGCGTGTCAT‑3' GAPDH F: 5'‑GGAAAGCTGTGGCGTGAT‑3' R: 5'‑AAGGTGGAAGAATGGGAGTT‑3' hsa, homo sapiens; miR, microRNA; CD44, CD44 molecule (Indian blood group); PCNA, proliferating cell nuclear antigen; -

Program in Human Neutrophils Fails To
Downloaded from http://www.jimmunol.org/ by guest on September 25, 2021 is online at: average * The Journal of Immunology Anaplasma phagocytophilum , 20 of which you can access for free at: 2005; 174:6364-6372; ; from submission to initial decision 4 weeks from acceptance to publication J Immunol doi: 10.4049/jimmunol.174.10.6364 http://www.jimmunol.org/content/174/10/6364 Insights into Pathogen Immune Evasion Mechanisms: Fails to Induce an Apoptosis Differentiation Program in Human Neutrophils Dori L. Borjesson, Scott D. Kobayashi, Adeline R. Whitney, Jovanka M. Voyich, Cynthia M. Argue and Frank R. DeLeo cites 28 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2005/05/03/174.10.6364.DC1 This article http://www.jimmunol.org/content/174/10/6364.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* • Why • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2005 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 25, 2021. The Journal of Immunology Insights into Pathogen Immune Evasion Mechanisms: Anaplasma phagocytophilum Fails to Induce an Apoptosis Differentiation Program in Human Neutrophils1 Dori L. -

Oncogenic ETS Fusions Deregulate E2F3 Target Genes in Ewing Sarcoma and Prostate Cancer
Downloaded from genome.cshlp.org on October 4, 2021 - Published by Cold Spring Harbor Laboratory Press Research Oncogenic ETS fusions deregulate E2F3 target genes in Ewing sarcoma and prostate cancer Sven Bilke,1,4 Raphaela Schwentner,2,4 Fan Yang,1 Maximilian Kauer,2 Gunhild Jug,2 Robert L. Walker,1 Sean Davis,1 Yuelin J. Zhu,1 Marbin Pineda,1 Paul S. Meltzer,1,5 and Heinrich Kovar2,3 1Genetics Branch, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland 20892, USA; 2Children’s Cancer Research Institute, St. Anna Kinderkrebsforschung, 1090 Vienna, Austria; 3Department of Pediatrics, Medical University, 1090 Vienna, Austria Deregulated E2F transcription factor activity occurs in the vast majority of human tumors and has been solidly implicated in disturbances of cell cycle control, proliferation, and apoptosis. Aberrant E2F regulatory activity is often caused by impairment of control through pRB function, but little is known about the interplay of other oncoproteins with E2F. Here we show that ETS transcription factor fusions resulting from disease driving rearrangements in Ewing sarcoma (ES) and prostate cancer (PC) are one such class of oncoproteins. We performed an integrative study of genome-wide DNA-binding and transcription data in EWSR1/FLI1 expressing ES and TMPRSS2/ERG containing PC cells. Supported by promoter activity and mutation analyses, we demonstrate that a large fraction of E2F3 target genes are synergistically coregulated by these aberrant ETS proteins. We propose that the oncogenic effect of ETS fusion oncoproteins is in part mediated by the disruptive effect of the E2F–ETS interaction on cell cycle control. Additionally, a detailed analysis of the regulatory targets of the characteristic EWSR1/FLI1 fusion in ES identifies two functionally distinct gene sets. -

Functional Genomics Analyses of RNA-Binding Proteins Reveal the Splicing Regulator SNRPB As an Oncogenic Candidate in Glioblastoma Bruna R
Correa et al. Genome Biology (2016) 17:125 DOI 10.1186/s13059-016-0990-4 RESEARCH Open Access Functional genomics analyses of RNA-binding proteins reveal the splicing regulator SNRPB as an oncogenic candidate in glioblastoma Bruna R. Correa1,2, Patricia Rosa de Araujo2, Mei Qiao2, Suzanne C. Burns2, Chen Chen3, Richard Schlegel3, Seema Agarwal3, Pedro A. F. Galante1* and Luiz O. F. Penalva2,4* Abstract Background: Glioblastoma (GBM) is the most common and aggressive type of brain tumor. Currently, GBM has an extremely poor outcome and there is no effective treatment. In this context, genomic and transcriptomic analyses have become important tools to identify new avenues for therapies. RNA-binding proteins (RBPs) are master regulators of co- and post-transcriptional events; however, their role in GBM remains poorly understood. To further our knowledge of novel regulatory pathways that could contribute to gliomagenesis, we have conducted a systematic study of RBPs in GBM. Results: By measuring expression levels of 1542 human RBPs in GBM samples and glioma stem cell samples, we identified 58 consistently upregulated RBPs. Survival analysis revealed that increased expression of 21 RBPs was also associated with a poor prognosis. To assess the functional impact of those RBPs, we modulated their expression in GBM cell lines and performed viability, proliferation, and apoptosis assays. Combined results revealed a prominent oncogenic candidate, SNRPB, which encodes core spliceosome machinery components. To reveal the impact of SNRPB on splicing and gene expression, we performed its knockdown in a GBM cell line followed by RNA sequencing. We found that the affected genes were involved in RNA processing, DNA repair, and chromatin remodeling.