Topical Emulsion Containing Lavandula Stoechas Essential Oil As a Therapeutic Agent for Cutaneous Wound Healing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plants for Landscapes
Plus 10 Water-Saving Tips for your Garden your for Tips Water-Saving 10 Plus 5 Printed on recycled paper recycled on Printed © 2014 San Diego County Water Authority Water County Diego San 2014 © sdbgarden.org sdbgarden.org thegarden.org tted landscape that looks beautiful and saves water. saves and beautiful looks that landscape tted retrofi or new a for ideas get Landscapes ese gardens are excellent places to to places excellent are gardens Th ese Cajon. El in Garden Conservation Water the and Encinitas Many of the plants in this guide are labeled and on display at the San Diego Botanic Garden in in Garden Botanic Diego San the at display on and labeled are guide this in plants the of Many 0 WaterSmartSD.org Plants for for Plants Nifty agencies member 24 its and hese Nift y 50 plants have been selected because they are attractive, T oft en available in nurseries, non-invasive, easy to maintain, long- term performers, scaled for residential landscapes and, once estab- lished, drought-tolerant. In fact, these plants thrive in San Diego’s semi- arid climate and can help restore regional authenticity to your home. What’s exciting is that authentic also means sustainable. Plants native to Mediterranean climate zones love it here as much as you do. Th ey adapted over thousands of years, and the animal species that depend on them for food and habitat adapted, too. In fact, there are thousands of ground cov- ers, grasses, succulents, perennials, shrubs, vines and trees to choose from. For more information, go to WaterSmartSD.org. -

J. APIC. SCI. Vol. 59 No. 2 2015 DOI: 10.1515/JAS-2015-0028
DOI: 10.1515/JAS-2015-0028 J. APIC. SCI. Vol. 59 No. 2 2015J. APIC. SCI. Vol. 59 No. 2 2015 Original Article FLORAL PHENOLOGY, NECTAR SECRETION DYNAMICS, AND HONEY PRODUCTION POTENTIAL, OF TWO LAVENDER SPECIES (LAVANDULA DENTATA, AND L. PUBESCENS) IN SOUTHWESTERN SAUDI ARABIA Adgaba Nuru* Ahmad A. Al-Ghamdi Yilma T. Tena Awraris G. Shenkut Mohammad J. Ansari Anwer Al-Maktary Engineer Abdullah Bagshan Chair for Bee Research, Department of Plant Protec- tion, College of Food and Agricultural Science, King Saud University Riyadh 11451 Riyadh (P. Box 2460), Saudi Arabia *corresponding author: [email protected] Received 18 August 2015; accepted 07 October 2015 A b s t r a c t The aim of the current study was to determine the floral phenology, nectar secretion dynamics, and honey production potentials of two naturally growing lavender species (L. dentata and L. pubescens), in southwestern Saudi Arabia. In both species, flowering is continuous. This means that, when open flowers on a spike are shaded, new flowers emerge. Such a flowering pattern might be advantageous to the plant to minimise competition for pollinators and promote efficient resource allocation. The flowering periods of the two species overlap. Both species secreted increasing amounts of nectar from early morning to late afternoon. The mean maximum volumes of accumulated nectar from bagged flowers occurred at 15:00 for L. pubescens (0.50 ± 0.24 µL/flower) and at 18:00 for L. dentata (0.68 ± 0.19 µL/flower). The volume of the nectar that became available between two successive measurements (three-h intervals) varied from 0.04 µL/flower to 0.28 µL/flower for L. -
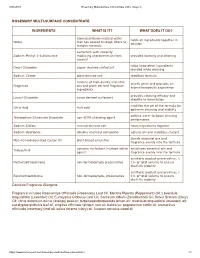
ROSEMARY MULTI-SURFACE CONCENTRATE INGREDIENTS WHAT IS IT? WHAT DOES IT DO? Contains Fragrance Allergens Fragrance Includes Rosm
9/24/2019 Rosemary Multi-Surface Concentrate | Mrs. Meyer's ROSEMARY MULTI-SURFACE CONCENTRATE INGREDIENTS WHAT IS IT? WHAT DOES IT DO? deionized/demineralized water holds all ingredients together in Water that has passed through filters to solution remove minerals surfactant with viscosity Sodium Methyl 2-Sulfolaurate modifying characteristics from provides foaming and cleaning coconut helps keep other ingredients Decyl Glucoside sugar- derived surfactant blended while cleaning Sodium Citrate plant-derived salt stabilizes formula mixture of high quality essential smells great and provides an Fragrance oils and plant-derived fragrance aromatherapeutic experience ingredients provides cleaning efficacy and Lauryl Glucoside sugar derived surfactant stability to formulation modifies the pH of the formula for Citric Acid fruit acid optimum cleaning and stability softens water to boost cleaning Tetrasodium Glutamate Diacetate non-EDTA chelating agent performance Sodium Sulfate mineral-derived salt holds ingredients together Sodium Hydroxide alkaline chemical compound adjusts pH and stabilizes chelant blends essential oils and PEG-40 Hydrogenated Castor Oil plant based emulsifier fragrance evenly into the formula nonionic surfactant (surface active solubilizes essential oils and Trideceth-9 agent) fragrance evenly into the formula synthetic product preservative, < Methylisothiazolinone non-formaldehyde preservative 1% of total volume to ensure shelf life stability synthetic product preservative, < Benzisothiazolinone non-formaldehyde, preservative -

Malacological Diversity on Four Lamiaceae in the Region of Tlemcen
Journal of Plant Sciences and Crop Protection Volume 1 | Issue 1 Review Article Open Access Malacological diversity on four Lamiaceae in the region of Tlemcen (Northwest of Algeria) Damerdji A* Department of Ecology and Environment, Faculty of SNV-STU, University of Tlemcen, Algeria *Corresponding author: Damerdji A, Department of Ecology and Environment, Faculty of SNV-STU, University of Tlemcen, 13000, Algeria, E-mail: [email protected] Citation: Damerdji A (2018) Malacological diversity on four Lamiaceae in the region of Tlemcen (North- west of Algeria). J Plant Sci Crop Protec 1(1): 106. doi: 10.15744/2639-3336.1.106 Received Date: March 07, 2018 Accepted Date: July 25, 2018 Published Date: July 27, 2018 Abstract The region of Tlemcen is located in the north-west Algeria. Tends arid climate leads to a degradation of vegetation in open formation, where are found the doum the diss and broom.... Other aromatic species are considered: rosemary, thyme, lavender and horehound. By their morphological and botanical four aromatic species belonging to the Labiatae family. We propose an approach to achieve diversity malacofauna identified on these Lamiaceae. These latters are certainly a nutritional source for this malacological fauna. For this, an inventory is made in different stations. Malacological wealth of thyme is estimated at 19, that the rosemary to 18, on 14 and lavender to last, that the horehound 7. It includes four families namely Milacidae the Sphincterochilidae the Helicidae and Subulinidae. Milacidae are present only in horehound and lavender stations. On the other hand, the Sphincterochilidae, namely Sphincterochila candidissima, is absent on horehound and lavander. -

Palynological Evolutionary Trends Within the Tribe Mentheae with Special Emphasis on Subtribe Menthinae (Nepetoideae: Lamiaceae)
Plant Syst Evol (2008) 275:93–108 DOI 10.1007/s00606-008-0042-y ORIGINAL ARTICLE Palynological evolutionary trends within the tribe Mentheae with special emphasis on subtribe Menthinae (Nepetoideae: Lamiaceae) Hye-Kyoung Moon Æ Stefan Vinckier Æ Erik Smets Æ Suzy Huysmans Received: 13 December 2007 / Accepted: 28 March 2008 / Published online: 10 September 2008 Ó Springer-Verlag 2008 Abstract The pollen morphology of subtribe Menthinae Keywords Bireticulum Á Mentheae Á Menthinae Á sensu Harley et al. [In: The families and genera of vascular Nepetoideae Á Palynology Á Phylogeny Á plants VII. Flowering plantsÁdicotyledons: Lamiales (except Exine ornamentation Acanthaceae including Avicenniaceae). Springer, Berlin, pp 167–275, 2004] and two genera of uncertain subtribal affinities (Heterolamium and Melissa) are documented in Introduction order to complete our palynological overview of the tribe Mentheae. Menthinae pollen is small to medium in size The pollen morphology of Lamiaceae has proven to be (13–43 lm), oblate to prolate in shape and mostly hexacol- systematically valuable since Erdtman (1945) used the pate (sometimes pentacolpate). Perforate, microreticulate or number of nuclei and the aperture number to divide the bireticulate exine ornamentation types were observed. The family into two subfamilies (i.e. Lamioideae: bi-nucleate exine ornamentation of Menthinae is systematically highly and tricolpate pollen, Nepetoideae: tri-nucleate and hexa- informative particularly at generic level. The exine stratifi- colpate pollen). While the -

Sharp's at Waterford Farm Your Neighborhood Farm Ask Us How To
Lemongrass – Essential for Thai Sharp’s at Waterford Herbs List cooking Farm Anise - Hyssop Lovage (Levistcum officinale) Farming in Howard County Basil Marjoram (Origanum majorana) since 1903 African Blue Amethyst Improved Purple Sweet Eleonora Zaatar, a hint of thyme, oregano & 4003 Jennings Chapel Rd. Elidia - Compact; container basil marjoram Brookeville, MD 20833 Genovese Golden - ornamental mostly Holy - Sacred Red and Green Tel: (410) 489-2572 Mint (Mentha sp.) Italian Large Leaf Chocolate Peppermint Lemon – Mrs. Burns www.sharpfarm.com Lemon Mint Mountain Mint Lettuce Leaf – Napoletano email: Peppermint Pineapple Mint Lime [email protected] Spearmint Sweet Thai Dark Opal Oregano (Origanum sp.) Red Rubin Greek Rutgers Devotion Zaatar ( a hint of thyme, oregano, & marjoram) Oreganum Syriaca) Borage: the herb of gladness Hot and Spicy - real tang, our favorite for adding to beans Catnip (Nepeta)- feline friends treat Parsley (Petroselinum crispum) Calendula, Neon Plain leaf (Italian or flat) Curly – double or triple Chamomile (German) Organic curled parsley (Bodegold) Italian Dark Green – Giant of Italy – huge leaves Your Neighborhood Chervil (Anthricus cerefolium) ‘crispum’ Vertissimo Farm Rosemary (Rosmarinus) Arp Chives (Allium) Hill Hardy Med Leaf (Purly) Ask Us How to Garden Salem Large leaf (staro) Sage (Salvia offincinalis) Helpful Hints: We pride ourselves Cilantro (Coriandrum sativium) Garden - Extrakta on knowing how to vegetable and herb Cruiser – more upright – great for Pineapple garden. Please ask if you need bunching – 50 days Savory Winter information on how to. Yields? Cutting Celery (Apium graveolens) Sorrel, French Spacing between plants? Staking? aka leaf celery When you plant, space your harvest Stevia (Stevia rebaudiana) by using varieties of different maturity Dill (Anethum graveolens): Nature’s natural sweetener dates. -

Medicinal Plants, Aromatic Herbs and Fragrance Plants in France
> Medicinal plants, aromatic herbs and fragrance plants in France: a small www.ihc2022.org but thriving sector with a strong traditional base and a dynamic research network Annabelle Bergoënd and Joséphine Piasentin In France, medicinal and aromatic plants paced growth. This production is very diverse small areas. Many producers reserve some (MAP) or herbal, medicinal and aromat- and dynamic, leaning on strong traditional areas as a side crop for their main produc- ic plants (HMAP) industries1 traditionally skills and benefiting from new techniques tion. Many of the plants are not referenced refer to culinary herbs, medicinal plants, and high value processing. With a growing in the European common agricultural pol- and plants cultivated for the fragrance and demand for natural products and unfolding icy (CAP) nomenclature. About 75% of the perfume industries. This agricultural sector opportunities for new markets, HMAP is fac- producers cultivate HMAPs to diversify their encompasses several hundred plant species, ing exciting prospects. production. The largest amount of land is as compared with field crops, such as cereals, occupied by fragrance plants, then medici- which may include single species grains. Characteristics of French HMAP nal plants. Aromatic herbs account for less The organic production for these crops is than 10% of the HMAP cultivated area. In significantly larger than traditional crops of Farm features and production are 2018, the estimated average farm was 4 ha global French agriculture. Wild harvest of geographically diverse for MAP production and 17 ha for perfume HMAP continues and is key for a success- The complexity of the production sector for plants. -

Antibacterial Effects of Citrus Limon and Lavandula Angustifolia Essential Oils
Journal of Applied Biological Sciences E-ISSN: 2146-0108 14(3): 358-364, 2020 Research Article ANTIBACTERIAL EFFECTS OF CITRUS LIMON AND LAVANDULA ANGUSTIFOLIA ESSENTIAL OILS Naima Sahraoui1, Meriem Djeghboub2*, Khelaf Saidani1, Souad Djeghboub1, 2 Jean Luc Hornick 1Veterinary sciences institute of Blida 1 university, Algeria PO box 270, Soumaa road, Blida – Algeria 2Department of Animal Resources Management, Faculty of Veterinary Medicine, University of Liège, FARAH Center, Quartier Vallée 2, Avenue de Cureghem 6, B43a, 4000 Liège, Belgium *Corresponding Author: E-mail: [email protected] (Received 14th January 2020; accepted 06th April 2020) ABSTRACT. Essential oils have been largely used for their antibacterial, antifungal and insecticidal properties. Thus this study evaluated the in vitro antibacterial activity of essential oils from lemon and lavender on 30 bacterial strains of Escherichia coli and Staphylococcus aureus either isolated from animals (cows and sheep) or obtained from reference strains provided by the Biotechnology and Animal Reproduction from the Laboratory of the Institute of Veterinary Sciences from Blida. The sensitivity of the strains was tested in vitro by aromatogram, using the two essential oils separately as well as their associations. Our results showed that lemon essential oil had a bacteriostatic effect on the tested strains of E. coli, while a high percentage of them was extremely sensitive to lavender oil, and more than half ones were extremely sensitive to the oil blend at a concentration of 100µl/disc. As for S. aureus, all the tested strains were extremely sensitive to the two essential oils as well as to their association. Lavender and/or lemon essential oils proved effective against strains of E. -

Evaluation of Antimicrobial Activity and Activity on the Autonomic Nervous
Progress in Nutrition 2019; Vol. 21, N. 3: 584-590 DOI: 10.23751/pn.v21i3.8385 © Mattioli 1885 Original article Evaluation of antimicrobial activity and activity on the autonomic nervous system of the lavender essential oils from Montenegro Svetlana Perovic1, Snezana Pantovic2, Valentina Scepanovic1, Andrej Perovic1, Vladimir Zivkovic3, Biljana Damjanovic-Vratnica4 1Department of Biology, Faculty of Natural Science and Mathematics, University of Montenegro, Dz. Vasingtona bb, Podgorica 2Faculty of Medicine, University of Montenegro, Krusevac bb, Podgorica - E-mail: [email protected]; 3Center for Eco- toxicological Research Podgorica, Boulevard Sarla de Gola 2, Podgorica; 4Faculty of Metallurgy and Technology, University of Montenegro, Dz. Vasingtona bb, Podgorica Summary. This study investigates chemical composition and antimicrobial activity of essential oil of Lavandula officinalis from Montenegro, Mediterranean, and effects on autonomic nervous system. The oil was analysed by GC-MS technique in order to determine the majority compounds while dilution method was used to determine minimal inhibitory concentration. The present study assessed autonomic parameters such as blood pressure, heart rate, and skin temperature to determine the arousal level of the autonomic nervous system. In addition, subjects were asked to estimate their mood responses such as feeling pleasant or unpleasant, uncomfortable, sen- suality, relaxation, or refreshing in order to assess subjective behavioural arousal. Chemical analysis by GC-MS identified 31 compounds in lavender oil representing 96.88% of the total oil. Linalool (24.84%), was a major component, together with linalyl acetate (22.39%), 1,8 cineole (18.13%) and camphor (12.88%). The investigat- ed lavender oil consisted mostly of oxygenated monoterpenes (87.95%) and monoterpene hydrocarbons (7%). -

The Chromosome-Based Lavender Genome Provides New Insights Into
Li et al. Horticulture Research (2021) 8:53 Horticulture Research https://doi.org/10.1038/s41438-021-00490-6 www.nature.com/hortres ARTICLE Open Access The chromosome-based lavender genome provides new insights into Lamiaceae evolution and terpenoid biosynthesis Jingrui Li1,2, Yiming Wang 3,YanmeiDong1,2, Wenying Zhang1,2,DiWang1, Hongtong Bai1,KuiLi3,HuiLi1 and Lei Shi1 Abstract The aromatic shrub Lavandula angustifolia produces various volatile terpenoids that serve as resources for essential oils and function in plant-insect communication. To better understand the genetic basis of the terpenoid diversity in lavender, we present a high-quality reference genome for the Chinese lavender cultivar ‘Jingxun 2’ using PacBio and Hi-C technologies to anchor the 894.50 Mb genome assembly into 27 pseudochromosomes. In addition to the γ triplication event, lavender underwent two rounds of whole-genome duplication (WGD) during the Eocene–Oligocene (29.6 MYA) and Miocene–Pliocene (6.9 MYA) transitions. As a result of tandem duplications and lineage-specific WGDs, gene families related to terpenoid biosynthesis in lavender are substantially expanded compared to those of five other species in Lamiaceae. Many terpenoid biosynthesis transcripts are abundant in glandular trichomes. We further integrated the contents of ecologically functional terpenoids and coexpressed terpenoid biosynthetic genes to construct terpenoid-gene networks. Typical gene clusters, including TPS-TPS, TPS- CYP450, and TPS-BAHD, linked with compounds that primarily function as attractants or repellents, were identified by fl 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; their similar patterns of change during ower development or in response to methyl jasmonate. Comprehensive analysis of the genetic basis of the production of volatiles in lavender could serve as a foundation for future research into lavender evolution, phytochemistry, and ecology. -

Chemical Composition and Acaricidal Effects of Essential Oils of Foeniculum Vulgare Mill
Hindawi Publishing Corporation Psyche Volume 2014, Article ID 424078, 6 pages http://dx.doi.org/10.1155/2014/424078 Research Article Chemical Composition and Acaricidal Effects of Essential Oils of Foeniculum vulgare Mill. (Apiales: Apiaceae) and Lavandula angustifolia Miller (Lamiales: Lamiaceae) against Tetranychus urticae Koch (Acari: Tetranychidae) Asgar Ebadollahi,1 Jalal Jalali Sendi,1 Alireza Aliakbar,2 and Jabraeil Razmjou3 1 Department of Plant Protection, Faculty of Agricultural Sciences, University of Guilan, Rasht 416351314, Iran 2Department of Chemistry, Faculty of Basic Sciences, University of Guilan, Rasht, Iran 3Department of Plant Protection, Faculty of Agriculture, University of Mohaghegh Ardabili, Ardabil, Iran Correspondence should be addressed to Asgar Ebadollahi; [email protected] Received 17 September 2014; Revised 14 November 2014; Accepted 24 November 2014; Published 14 December 2014 Academic Editor: Nguya K. Maniania Copyright © 2014 Asgar Ebadollahi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Utilization of synthetic acaricides causes negative side-effects on nontarget organisms and environment and most of the mite species such as two spotted spider mite, Tetranychus urticae Koch, are becoming resistant to these chemicals. In the present study, essential oils of fennel, Foeniculum vulgare Mill., and lavender, Lavandula angustifolia Miller, were hydrodistilled using Clevenger apparatus and chemical composition of these oils was analyzed by GC-MS. Anethole (46.73%), limonene (13.65%), and -fenchone (8.27%) in the fennel essential oil and linalool (28.63%), 1,8-cineole (18.65%), and 1-borneol (15.94%) in the lavender essential oil were found as main components. -

Topped Lavender Lavandula Stoechas
Source: http://keyserver.lucidcentral.org/weeds/data/080c0106-040c-4508-8300-0b0a06060e01/media/html/ Lavandula_stoechas.htm Downloaded 27/01/2016 Topped lavender Lavandula stoechas Scientific Name Lavandula stoechas L. Synonyms Lavandula stoechas L. subsp. stoechas Common Names bush lavender, French lavender, Italian lavender, Spanish lavender, top lavender, topped lavender, wild lavender Family Labiatae (South Australia) Lamiaceae (Queensland, New South Wales, the ACT, Victoria, Tasmania, Western Australia and the Northern Territory) Origin Native to north-western Africa (i.e. Algeria, Morocco and Tunisia), the Madeira Islands, the Canary Islands, southern Europe (i.e. Greece, Italy, France, Portugal and Spain) and western Asia (i.e. Cyprus, Israel, Lebanon, Syria and Turkey). Naturalised Distribution Widely naturalised in southern Australia (i.e. in some parts of central and southern New South Wales, in the ACT and Victoria, in eastern South Australia and in south-western Western Australia). Also naturalised in New Zealand and south-western USA (i.e. California). Cultivation Widely cultivated in the temperate regions of Australia. Numerous cultivars are currently available, including several modern ones that do not set seeds (e.g. 'Avonview', 'Fairy Wings', 'Pukehou', 'Merle', 'Kew Red', 'Marshwood' and 'Ploughman's Purple'). Many of these cultivars are actually hybrids involving this and other Lavandula species. Habitat A weed of pastures, open woodlands, roadsides, disturbed sites, gardens, waste areas and waterways mainly in the temperate regions of Australia. Distinguishing Features . a low-growing shrub less than 1 m tall. its stems are square in cross-section and densely covered in greyish hairs. its long and narrow leaves are oppositely arranged, stalkless, and have their margins curved downwards.