Supplementary Materials New Urea Derivatives As Potential
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vijayawada, Management Authority and Moted Without Appearing in Committee, Who Was Admitted North Andhra
!"# $%& !"#$% & % '!'" $(' & !&$)' '$" '!$ '& !" #$#%&#' #)**+%,+ !! " !#$#%!& '() . 9 : 1 ; 49 8 0 + <<0 4 ; 43 8 4 < 40?@?@ <+ (99 1 + B; 8 4 ; < Q> 8-A ? *470 #8940#&4.,44.$4%3#3&++#0'4; #470.#+3#0*;..#4=&4;.4#3,%8& 14'0#.4'.,C%.4.&%.#'.)&$0&44'&C*$%0 & .40.#$8.).4+ 4#00&0*.0*.43& 434'&0#%.#36,#14'4$#4,.8,0&++3+.*84& )#04346.#'4.84.,&.4.$#3&88.#++$ ,8483.$+0;& #00 .$4%+.)43.4#303& .88.4 3800.40;.# ;8<%%.#34'.4 1%10.))+3#0&*#%4&.#+370$+#'0 +>$&#).#8#)8+&.84%4#+ .43.4<& 830&.$+33&#.&4.#80.)C.$#4+0%%.43$%4%.#4'%00 .))%+4.)%.4; 8++4.,%.43$%3.4 %.4.8<,03&00$))#4'#%$##4%.)& +$ +>$0%&8;#$4,& 4'+0&#' $43#40'.44303034..%##.$0.4D*#+++#+D<& *8,#"< #.$0+; 00%&3$+3).#*8,#"< $+%#%.+0&).#%3&8.%$0&.#)#0 %&#;&.4.$#04.,+*.*+43.#'40.40 830&.$+34.4##&0.#4844#0.0.43$% &3%0.4.*.0*.4& 8&0,4144&# #84.))%4303&&4.+.4'#)+,+. &.#%.44%3&*#%$+#,$+34'0%#.00 &'4#+*$,+%.,+4&%.8*+%.)&*#0.4 84'.)&%$%.88.4 %.44$0*#8840#<&+0*%$+.4,.$ .43.4<=&470*+>$&0'.4$*?.4# 43%3;& 03<& .$4%+03&04.3& $'$0"<4T';& ,70*.+%+)$$#&0,4'#.4'4#%4 4+..80,$#;�&+3,).#0&+)).#6 D30#00&%.#'.)&++'30$%3,)+8%.#, %$%.88..14.%.403#.4 10;)#&%03&.0*+).#$40*%)3 .%%$*3#4%4@!?04$43#%.##3. 8483.$+004.+.4.)&4.#80.)C.$#4+0% &.4'.4' -6@!0$.443& &+&%&%10;�'4.44.4&+00 .*#.#).##4770*%+ *#.40 %.43$%D43;&#).#;300&83.3&#.& 0$,0>$4+.%13.44Ʈ %800$#*#0< %$A B< 4.#80)#83,< 4'+< -.-/#"$ 01. -

UFO Digital Cinema THEATRE COMPANY WEB S.No
UFO Digital Cinema THEATRE COMPANY WEB S.No. THEATRE_NAME ADDRESS CITY ACTIVE DISTRICT STATE SEATING CODE NAME CODE 1 TH1011 Maheshwari 70Mm Cinema Road,4-2-198/2/3, Adilabad 500401 Adilabad Y Adilabad ANDHRA PRADESH UFO 698 2649 2 TH1012 Sri Venkataramana 70Mm Sirpur Kagzahnagar, Adilabad - 504296 Kagaznagar Y Adilabad ANDHRA PRADESH UFO 878 514 3 TH1013 Mayuri Theatre Mancherial, Adilabad, Mancherial - 504209, AP Mancherial Y Adilabad ANDHRA PRADESH UFO 354 1350 4 TH1014 Noor Jahan Picture Palace (Vempalli) Main Road, Vempalli, Pin- 516329, Andhar Pradesh Vempalli Y Adilabad ANDHRA PRADESH UFO 635 4055 5 TH1015 Krishna Theatre (Kadiri) Dist. - Ananthapur, Kadiri - 515591 AP Anantapur Y Anantapur ANDHRA PRADESH UFO 371 3834 Main Road, Gorantla, Dist. - Anantapur, Pin Code - 6 TH1016 Ramakrishna Theatre (Gorantla) Anantapur Y Anantapur ANDHRA PRADESH UFO 408 3636 515231 A.P 7 TH1017 Sri Varalakshmi Picture Palace Dharmavaram-515671 Ananthapur Distict Dharmavaram Y Anantapur ANDHRA PRADESH UFO 682 2725 8 TH1018 Padmasree Theatre (Palmaner) M.B.T Road, Palmaner, Chittor. Pin-517408 Chittoor Y Chittoor ANDHRA PRADESH UFO 587 3486 9 TH1021 Sri Venkateswara Theatre Chitoor Vellore Road, Chitoor, Dist Chitoor, AP Chittoor Y Chittoor ANDHRA PRADESH UFO 584 2451 10 TH1022 Murugan Talkies Kuppam, Dist. - Chittoor, AP Kuppam Y Chittoor ANDHRA PRADESH UFO 316 3696 Nagari, Venkateshmudaliyar St., Chittoor, Pin 11 TH1023 Rajeswari Theatre Nagari Y Chittoor ANDHRA PRADESH UFO 600 1993 517590 12 TH1024 Sreenivasa Theatre Nagari, Prakasam Road, Chithoor, -
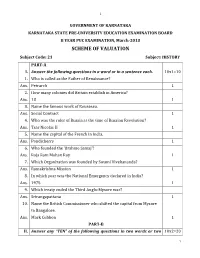
SCHEME of VALUATION Subject Code: 21 Subject: HISTORY PART-A I
1 GOVERNMENT OF KARNATAKA KARNATAKA STATE PRE-UNIVERSITY EDUCATION EXAMINATION BOARD II YEAR PUC EXAMINATION, March-2013 SCHEME OF VALUATION Subject Code: 21 Subject: HISTORY PART-A I. Answer the following questions in a word or in a sentence each. 10x1=10 1. Who is called as the Father of Renaissance? Ans. Petrarch 1 2. How many colonies did Britain establish in America? Ans. 13 1 3. Name the famous work of Rousseau. Ans. Social Contract 1 4. Who was the ruler of Russia at the time of Russian Revolution? Ans. Tsar Nicolas II 1 5. Name the capital of the French in India. Ans. Pondicherry 1 6. Who founded the ‘Brahmo Samaj’? Ans. Raja Ram Mohan Roy 1 7. Which Organization was founded by Swami Vivekananda? Ans. Ramakrishna Mission 1 8. In which year was the National Emergency declared in India? Ans. 1975 1 9. Which treaty ended the Third Anglo-Mysore war? Ans. Srirangapattana 1 10. Name the British Commissioner who shifted the capital from Mysore to Bangalore. Ans. Mark Cubbon 1 PART-B II. Answer any “TEN” of the following questions in two words or two 10x2=20 1 2 sentences each: 11. Who circumnavigated the earth for the first time? Which country did he belong to? Ans. Ferdinand Magellan– Portugal / Spain 1+1 12. Mention the three important watchwords of French Revolution. Ans. Liberty, Equality and Fraternity. 1+1 13. Name the architect of German Unification. Which policy did he follow? Ans. Bismark – Blood & Iron. 1+1 14. What Is ‘Great Leap Forward’? Who introduced it? Ans. -

Role of Haveri District Leaders in the National Movement – a Case Study
© 2019 JETIR June 2019, Volume 6, Issue 6 www.jetir.org (ISSN-2349-5162) Role of Haveri District Leaders in the National Movement – A case study * Mamata Sawakar, Asst Professor, Dept of Political Science, Govt First Grade College,Ranebennur **Dr.Prasannakumar.K, Asst Professor, Dept of Political Science, Sahyadri Arts & Commerce College, Shimoga. Abstract Haveri district is exactly in the center of Karnataka with equal distance from Bidar in the far north to Kollegal in the far south. It is also known as the gateway district to the northern districts of Karnataka. Haveri district has a very rich culture and tradition. The district is proud to be the birth place of Santa Shishunala Sharif, great saint Kanakadasaru, Sarvagnya, Hanagal Kumara Shivayogigalu, Wagish Panditaru, Writer Galaganatharu, Ganayogi Panchakshari Gavayigalu, Gnyana Peetha Awardee Dr.V.K.Gokak and many more. The freedom fighter Mailara Mahadevappa, who resisted British rule, is from Motebennur in Haveri District. Another freedom fighter Gudleppa Hallikere a native of Hosaritti is also from this district. He started a residential school Gandhi Grameen Gurukul in Hosaritti. During the Quit India Movement, Gandhiji gave the clarion call ‘Do or die’. A great movement to oust the British was unleashed. Mahadevappa lead the movement of South division of Dharwad. He started Kara Nirakarane i.e. refusal to pay land revenue movement. He encouraged farmers not to pay land revenue to the Government. This movement spread like wild fire. The lands of those peasants who did not pay taxes were confiscated. Meanwhile, some peasants joined hands with the British and purchased those confiscated lands. -

Government of Karnataka Provisional Ward Wise
Government of Karnataka O/o Commissioner for Public Instruction, Nrupatunga Road, Bangalore - 560001 Provisional Ward wise Neighbourhood Schools - 2016 ( URBAN ) Ward Code School Code Management Lowest High Entry type class class class Ward Name School Name Medium Sl.No. District: Gulbarga Block : ALAND Ward Name : SANGOLAGI [G]---290401108 290401108 29040110808 Pvt Unaided 1 7 Class 1 SANGOLAGI [G] SANGOLLI RAYANNA HPS SANGOLIGI (G) 05 - Kannada 1 290401108 29040110809 Pvt Unaided 1 5 Class 1 SANGOLAGI [G] S.R.GUTTEDAR PETROL BUNK 05 - Kannada 2 Ward Name : WARD NO 1---290401152 290401152 29040115201 Govt. 1 7 Class 1 WARD NO 1 GOVT HPS SULTANPUR GALLI ALAND 05 - Kannada 3 290401152 29040115203 Govt. 1 7 Class 1 WARD NO 1 GOVT GIRLS HPS (K) ALAND 05 - Kannada 4 290401152 29040115204 Pvt Aided 1 7 Class 1 WARD NO 1 VISHWESHWARAYYA HPS (K) 05 - Kannada 5 290401152 29040115202 Pvt Aided 1 7 Class 1 WARD NO 1 AL-FAROOQ HPS ALAND 18 - Urdu 6 Ward Name : WARD NO 2---290401153 290401153 29040115301 Govt. 1 7 Class 1 WARD NO 2 GOVT GHPS ALAND ( U ) 18 - Urdu 7 Ward Name : WARD NO 4---290401155 290401155 29040115501 Pvt Aided 1 7 Class 1 WARD NO 4 DJHPS ALAND 10 - Marathi 8 290401155 29040115504 Pvt Unaided 1 7 Class 1 WARD NO 4 KUVEMPU HPS ALAND 05 - Kannada 9 290401155 29040115505 Pvt Unaided 1 5 Class 1 WARD NO 4 LITTLE ENJALS LPS ALAND 05 - Kannada 10 290401155 29040115508 Pvt Unaided 1 5 Class 1 WARD NO 4 SHRI SIDDARAMESHWAR LPS ALAND 05 - Kannada 11 290401155 29040115510 Pvt Unaided 1 5 Class 1 WARD NO 4 SHRI GURU SCHOOL BALAJI NAGAR 05 - Kannada 12 290401155 29040115512 Pvt Unaided 1 5 Class 1 WARD NO 4 NETAJI LPS ALAND 05 - Kannada 13 Ward Name : WARD NO 5---290401156 290401156 29040115601 Govt. -

Haveri District and the Indian Freedom Movement
© 2019 JETIR August 2019, Volume 6, Issue 8 www.jetir.org (ISSN-2349-5162) Haveri District and the Indian freedom movement * Mamata Sawakar, Asst Professor, Dept of Political Science, Govt First Grade College,Ranebennur **Dr.Prasannakumar.K, Asst Professor, Dept of Political Science, Sahyadri Arts & Commerce College, Shimoga. Abstract The one great blessing that the unification of India through the British rule had brought about was the rise and growth of a sense of national unity, however vague and uncertain it might have been in the initial stages; and this conception was strengthened by the work of reform bodies like the Brahmo Samaj, Ramakrishna Mission ( established by Swami Vivekananda), the Prarthana Samaj in Bombay (1867), the Arya Samaj in the Punjab (1875), the Theosophical Society in Madras (1882), and the Sarvajanik Sabha of Poona. Most of these movements aimed of course at the eradication of social abuses and unhealthy developments in religious practices. But this renaissance, especially in India where every aspect of life is infused by religious and moral purposes, was bound to lead to efforts at improvement in the political field also. The political revival of the Maratha and Sikh powers was preceded by religious movements, rousing the people to new endeavour. The press and the new literature that grew up in the years previous to 1885 also indicated the disaffection that was steadily growing among the people. Haveri district along with Gadag district was earlier part of undivided Dharwad district. Owing to the demands of the people Haveri district was carved out of old Dharwad district and was formed on 24.08.1997. -

Incomplete Statements. Choose the Correct Answer and Shade the Correct Choice in the OMR Given to You with Blue/Black Ball Point Pen
. Four choices are given for each of the questions/ incomplete statements. Choose the correct answer and shade the correct choice in the OMR given to you with blue/black ball point pen. 1. Gateway of European Trade was A. London. B. Constantinople. C. Paris. D. New Delhi Ans: B. Constantinople. 2. Ottoman Turks captured city of Constantinople in the year A. 1435. B. 1455. C. 1434. D. 1453. Ans: D. 1453. 3. Vasco da Gama discovered new sea route to India in A. 1492. B. 1498. C. 1453. D. 1499. Ans: B. 1498. 4. The first Europeans who came to India for trade were A. French. B. British. C. Dutch. D. Portuguese. Ans: D. Portuguese. 5. Francisco de Almeida implemented 'Blue Water Policy' to A. Establish supremacy over land. B. Establish supremacy over the Sea. C. Establish supremacy over sky. D. Establish supremacy over both land and Sea. Ans: B. Establish supremacy over the Sea. 6. The real founder of Portuguese Empire in India was A. Vasco da Gama. B. Alfonso de Albuquerque. C. Almeida. D. Robert Clive. Ans: B. Alfonso de Albuquerque 7. Albuquerque occupied the Goa in 1510 from A. British. B. Bijapur Sultans. C. Hoysalas. D. Kakatiyas. Ans: B. Bijapur Sultans. 8. British East India Company was established in the year A. 1602. B. 1600. C. 1664. D. 1498. Ans: B. 1600. 9. British ambassador who got trade permission from Mughal emperor Jahangir was A. Robert Clive. B. Thomas Roe. C. Charles II. D. James I. Ans: B. Thomas Roe. 10. Fort built by British in Madras was A. -

List of Candidates of Ssc(T)-49 Course Selection Centre South Bangalore
LIST OF CANDIDATES OF SSC(T)-49 COURSE SELECTION CENTRE SOUTH BANGALORE (CIVIL- 080-25588065) 1. Candidates are required to report at Krantivira Sangolli Rayanna (KSR) Bangalore (SBC) Railway Station at 1400 hours (2 PM) on the dates as specified against each for SSB Interviews for the Subject Course scheduled to be held at Selection Centre South Bangalore. They are required to bring with them the following documents in ORIGINAL alongwith two photocopies each duly attested :- (a) Passing certificate of matriculatioin / 10th standard exam for verification of age proof. (No other document except matriculation certificate in original is acceptable for age proof) (b) Mark sheet and certificate of 10th Class. (c) Mark sheets and certificates of 10+2 classes/ Intermediate. (d) All years/ semesters mark sheets of graduation. (e) Degree/ Provisional Degree. (f) If you are studying in final year of degree course, then please bring a certificate from the principal of your college / institution stating that :- Certified that Shri _____________________ S/o ______________________ is a Bonafide student of this University / College studying in final year / semester of Graduation degree of ____________________ (Branch). His final year / “It is certified that ______________ s/o Shri _________________ is a bonafide student of ________ (School/ College) and is presently studying in _____________. His final board/ semester examination will be over before 01 Oct 2017. Place : (Signature of the Principal/Registrar of the Date : the College/University where studying with stamp) Important Note :- Final Year Engineering degree candidates must complete all the formalities including written, practicals, projects, backlogs, viva voce, etc for award of qualifying BE/ B.Tech Degree before 01 Oct 2017. -

KSLU's Law School
KSLU’s Law School Sports Annual Report 2015-16 KARNATAKA STATE LAW UNIVERSITY’s LAW SCHOOL Navanagar, Hubballi-580025. Sports Section, Phone 0836-2220024 E-mail: [email protected] Team Participated in Cross Country (Men/Women) inter collegiate tournament cum selection held at Vivekananda Law College, Puttur from 19th to 20th September 2015. Team Participated in Chess inter collegiate tournament cum selection held at S. D. M. Law College, Mangalore from 21st to 22nd September 2015. Team Participated in Football (Men) inter collegiate tournament cum selection held at M. S. Ramaiah Law College, Bangalore from 06th to 07th November 2015. Team Participated in Cricket (Men) inter collegiate tournament held at Vaikunta Baliga Law College, Udupi from 17th to 21th November 2015. Team Participated in THROWBALL and TENNIKOIT (Women) inter collegiate tournament held at Sharada Vilas Law College, Mysore from 22nd to 23rd March 2016. Team Participated in Hubballi Zonal Athletic Meet Selections (Men and Women) inter collegiate Competition held at Hukadli Ajja Law College, Dharwad on 26th April 2016. National Sports Day was celebrated on the 29th August 2015. KSLU Sports Section organized a event: “WALK FOR HEALTH, JOG FOR FITNESS and RUN FOR COMPETITION”. In view of Birthday of Major Dhyan Chand Singh (Indian Hockey Player) for the students, teaching, non-teaching staff and statutory officers of KSLU. Team Participated in 4th Inter Collegiate Athletic Meet (Men and Women) Competition Organized by Karnataka State Law University, Hubballi held at R. N. Shetty Stadium, Dharwad from 11th to 12th May 2016. KSLU Law School 6th Annual Sports Meet in Indoor and Outdoor held at Karnatak Arts College, Dharwad on 30th March 2016. -

The Condemned Can't Fi
follow us: friday, january 24, 2020 Delhi City Edition thehindu.com 24 pages ț ₹10.00 facebook.com/thehindu twitter.com/the_hindu Pawan Varma is free Top UN court orders India begins T20I series Thiem and Wawrinka to leave JD(U) if he so Myanmar to prevent against New Zealand survive scares; Nadal wishes, says Nitish Rohingya genocide at Auckland today cruises into third round page 12 page 14 page 18 page 20 Printed at . Chennai . Coimbatore . Bengaluru . Hyderabad . Madurai . Noida . Visakhapatnam . Thiruvananthapuram . Kochi . Vijayawada . Mangaluru . Tiruchirapalli . Kolkata . Hubballi . Mohali . Malappuram . Mumbai . Tirupati . lucknow . cuttack . patna NEARBY CCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCC Virushit Wuhan locked down In full finery Trains and planes indefinitely suspended; travel curbs in neighbouring cities too Agence France-Presse Beijing Indian nurse ‘Peaceful protests will China on Thursday locked tests positive deepen democratic roots’ down some 20 million peo NEW DELHI ple at the epicentre of the An Indian nurse working at Referring to the ongoing new coronavirus outbreak, a hospital in Saudi Arabia agitation against the banning planes and trains tested positive for the novel amended citizenship law and a from leaving in an unprece Coronavirus when she and proposed nationwide National Register of Citizens exercise, dented move aimed at con nearly 100 of her Indian former President Pranab taining the disease, which colleagues, mostly from Mukherjee on Thursday said has spread to other coun Kerala, were screened, the that the “peaceful protests” tries. government said. She is would lead to a deepening of The respiratory virus, being treated at Aseer India’s democratic roots. He 2019nCoV, has claimed 17 On alert: A soldier uses a digital thermometer to check a National Hospital. -

Test Booklet Series-D
1.Consider the following statements 6.The Discovery Channel in it's special episode, featuring the famed survivalist and adventurer Bear Grylls with PM Modi, was shot in 1. Sangolli Rayanna was a prominent warrior from Karnataka. the Jim Corbett National Park and aired at 9pm on August 12. Name of the Special Episode is 2. He was the army chief of the Kingdom of Kittur ruled at the time by Rani Chennamma a. Man Vs Wild b. Man Vs Environment 3. He fought the British East India Company till his death. c. Man Vs Nature d. Man Vs World 4. The Bengaluru City Railway Station will soon be renamed after 19th century warrior Krantiveera Sangolli Rayanna 7.Match the following Dams & Rivers across India Which of the above statements are true? Dam River a 1,2, & 3 b 2,3 & 4 1. Sardar Sarovar Dam (2017) a. Kaveri c 1,3, & 4 d All 2 Krisna Raja Sagara (1938) b. Periyar 2.Dandi March took place from: 3. Idukki c. Narmada 4. Somasila d. Krishna a. March 12- April 6, 1930 b. March 12- April 6, 1931 5. Almatti e. Pennar c. March 12- April 6, 1932 d. March 12- April 6, 1929 3.During India's Freedom Struggle, which one of the following led to a. 1-a, 2-b, 3-c, 4-d, 5-e b.1-c, 2-a, 3-b, 4-e, 5-d the first 'All India Hartal'? c. 1-e, 2-d, 3-a, 4-b,5-c d. 1-d, 2-a, 3-c, 4-d, 5-e a. -

Author Title
Author Title - Jagattina Achharigalu Dr Mark A Garlick Atlas of The Universe Robert Burnham Children's Altas of the Universe Beverly McMillan The Illustrated Atlas of the Human Body - Atlas of the world Pam Robson and Mick Seller Encyclopedia of Science Projects NCERT Marigold Jyotsna Bharati Everlasting fables of hitopadesha Shree book Centre Vikram and Betal Shanthi Publication Best of Fairy tales PC Wren and H Martin High School English Grammar & Composition PC Wren and H Martin Key to high School English Grammar Council for the Indian School ICSE March 2018 Regulations and Syllabuses J D Murthy Contemporary English Grammar for scholars Future Aesop's fables Rajesh Kavassery Jataka Tales Dr Samir Kumar Ghosh A Textbook Of Practical Physics DR S N Ghoshal Nuclear Physics S CGarg Thermal Physics Dr H R Modi Handbook Chemistry O P Agarwal Advanced Practical Organic Chemistry Samir Kumar Ghosh A textbook of Advanced Practical Physics Book Palace Encyclopedia of The Living World K S Krishna Swamy Astrophysics - A modern Perspective Arthur Beiser Concepts of Modern Physics Arthur Beiser Fundamentals of Physics with Application - Physics - Handbook Key notes terms definition David Eagleman The Brain the story of You Amit Rastogi Mathematics-keynotes term definitions formule Alan Graham Mathematics A basic introduction David Halliday Principles of Physics M Balaram Walter Elias Disney Mary Joseph Thomas Alva Edison Prof. Gayatri Murthy Albert Einstein Dr. B R Suhas Louis Pasteur Dr . H S Niranjanaaradhya Charles Darwin Be. Go Ramesh Albert Schweitzer Rajeshwari Krishna Dr. Salim Ali Kaivaara Gopinath wright Sahodararu Dr. B R Suhas William Harvey Rajeshwari Krishna Abraham Lincoln Dr.